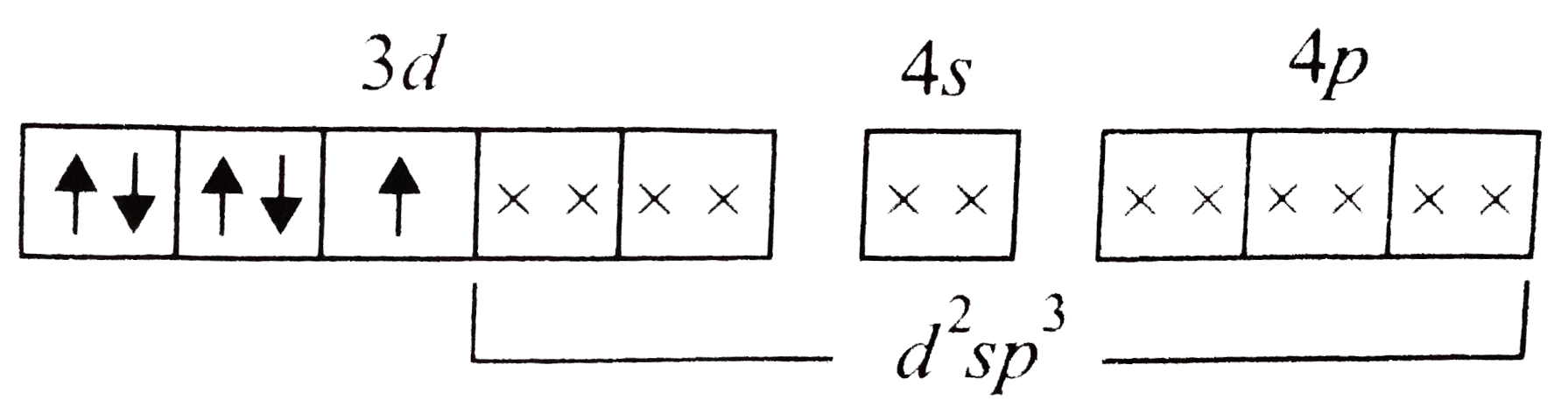A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
A2Z|Exercise Crystal Field Theory (Cft)|16 VideosCOORDINATION COMPOUNDS
A2Z|Exercise Metal Carbonyls, Stability Of Complexes And Applications|23 VideosCOORDINATION COMPOUNDS
A2Z|Exercise Optical Isomerism|22 VideosCHEMICAL KINETICS
A2Z|Exercise Section D - Chapter End Test|30 VideosELECTROCHEMISTRY
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-COORDINATION COMPOUNDS-Wemer Theory And Vbt
- The structure of iron pentacarbonyl is:
Text Solution
|
- [Cr(NH3)6]^(3+) ion is:
Text Solution
|
- In hexacyanidomanganate(II) ion the Mn atom assumes d^(2)sp^(3)- hybri...
Text Solution
|
- Which is true in the case of [Ni(CO)4] complex?
Text Solution
|
- Which one of the following is an outer orbital complex and exhibits pa...
Text Solution
|
- Which of the following complexes exhibits the highest paramagnetic beh...
Text Solution
|
- A magnetic moment of 1.73 B.M. will be shown by one among the followin...
Text Solution
|
- A square planar complex is formed by hybridization of which atomic orb...
Text Solution
|
- Among the following complexes : K(3)[Fe(CN)(6)],[Co(NH(3))(6)]Cl(3) , ...
Text Solution
|
- Consider the follwing complexes ion P,Q and R P =[FeF(6)]^(3-), Q=[V...
Text Solution
|
- Among the following ions which one has the highest paramagnetism?
Text Solution
|
- The reaction [Fe(CNS)6]^(3-)rarr[FeF6]^(3-) takes place with:
Text Solution
|
- dsp^2-hybridization is found in
Text Solution
|
- The primary valency of iron in K4[Fe(CN)6] is
Text Solution
|
- The magnetic moment of K3[Fe(CN)6] is found to be 1.7 B.M. How many un...
Text Solution
|
- [Cr(H2 O)6]Cl3 (at no. of Cr = 24) has a magnetic moment of 3.83 B.M. ...
Text Solution
|
- Which of the following complex is an outer orbital complex?
Text Solution
|
- The correct order of magnetic moments (spin values in B.M.) among is:
Text Solution
|
- Which of the following has the regular tetrahedral structure?
Text Solution
|
- Which one of the following has lowest value of paramagnetic behaviour?
Text Solution
|