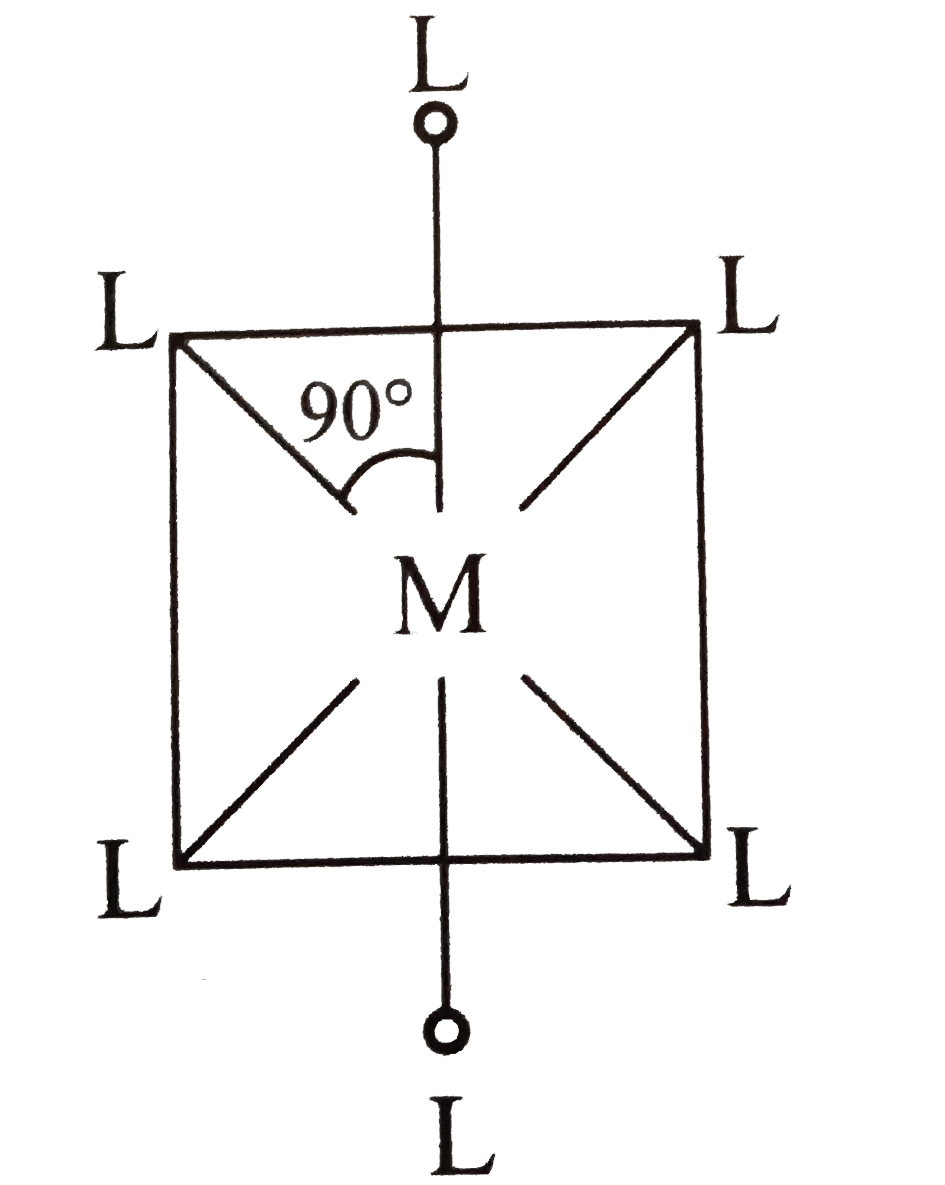
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
A2Z|Exercise Crystal Field Theory (Cft)|16 VideosCOORDINATION COMPOUNDS
A2Z|Exercise Metal Carbonyls, Stability Of Complexes And Applications|23 VideosCOORDINATION COMPOUNDS
A2Z|Exercise Optical Isomerism|22 VideosCHEMICAL KINETICS
A2Z|Exercise Section D - Chapter End Test|30 VideosELECTROCHEMISTRY
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-COORDINATION COMPOUNDS-Wemer Theory And Vbt
- Which of the following has the regular tetrahedral structure?
Text Solution
|
- Which one of the following has lowest value of paramagnetic behaviour?
Text Solution
|
- Velence bond theroy describes the bonding in complexs in terms of coor...
Text Solution
|
- Nickel (Z=28) combines with a uninegative monodentate ligand X^(-) to ...
Text Solution
|
- Which one of the following has a square planar geometry? (Co=27, Ni=...
Text Solution
|
- The octahedral complex of a metal ion M^(3+) with four monodentate lig...
Text Solution
|
- Which of the following facts about the complex [Cr(NH3)6]Cl3 is wrong?
Text Solution
|
- The magnetic moment (spin only) of [NiCl4]^(2+) is
Text Solution
|
- Which of the following is a low spin complex?
Text Solution
|
- Complexes with halide ligands are generally:
Text Solution
|
- Among the following ions, which one has the highest paramagnetism?
Text Solution
|
- Among [Ni(CO)4], [Ni(CN)4]^(2-), [NiCl4]^(2-) species, the hybridizati...
Text Solution
|
- Which is correct in the case of [NiCl4]^(2-) complex?
Text Solution
|
- The shape of [Cu(NH3)4]Cl2 is:
Text Solution
|
- For which transition metal ions are low spin complexes possible:
Text Solution
|
- An octahedral complex is formed when hybrid orbitals of the following ...
Text Solution
|
- The primary valence of the metal ion in the coordination compound K2[N...
Text Solution
|
- The number of unpaired electrons in the complex ion [CoF6]^(3-) is (At...
Text Solution
|
- The compound which does not show paramagnetism is
Text Solution
|
- When excess of ammonia is added to CuSO4 solution, the deep blue colou...
Text Solution
|