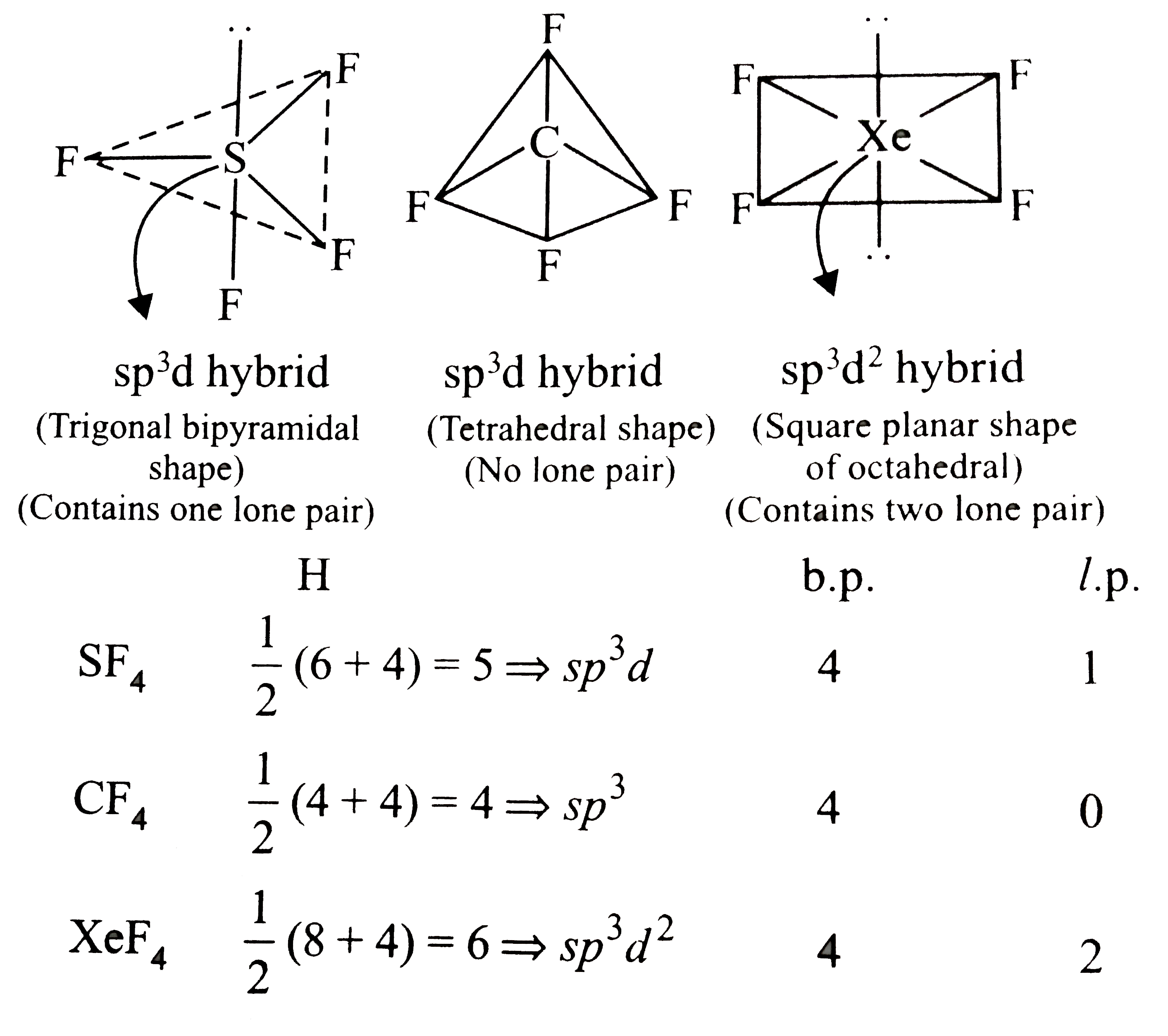A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise Molecular Orbitial Theory|42 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise Force Of Attraction|22 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise Valence Bond Theory (Vbt)|30 VideosATOMIC STRUCTURE
A2Z|Exercise Section D - Chapter End Test|30 VideosCHEMICAL EQUILIBRIUM
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Vsepr Theory And Hybridisation
- The molecule which has pyramidal shapes is:
Text Solution
|
- The type of hybrid orbitals used by the chlorine atom in CIO(2^(-)) is
Text Solution
|
- Molecular shapes of SF(4). And CF(4) and XeF(4) are:
Text Solution
|
- In which of the following compounds corban atom undergoes hybridisati...
Text Solution
|
- Which one of the following molecules is planar?
Text Solution
|
- The molencule which posses both sp^(3) and sp^(3)d^(2) hybridisation i...
Text Solution
|
- Which one of the following compounds has sp^(2) hybridization?
Text Solution
|
- Which of the following have linear shapes?
Text Solution
|
- A molecule XY(2) contains two sigma bonds two pi bond and one lone pai...
Text Solution
|
- The maximum number of 90^@ angles hetween bond pair -bond pair of ele...
Text Solution
|
- The correct order of hybridisation of the central atom in the followin...
Text Solution
|
- Which of the following has a 3 centred 2 electron bond?
Text Solution
|
- Which species has the maximum number of lone pair of electrons on the ...
Text Solution
|
- Among CIF(3), BF(3) and NH(3) molencules the one with non-planar geome...
Text Solution
|
- Specify the coordination geometry around and the hybridisation of N an...
Text Solution
|
- Which of the following molecules planer planar geometry?
Text Solution
|
- The two types of bonds present in B(2)H(6) are covalent and .
Text Solution
|
- Which has regular tetrahedral geometary ?
Text Solution
|
- Which of the following are isolectronic and iso-structural ? NO(3)^(...
Text Solution
|
- The percentage s-character of the hybrid orbitals in methane , ethene ...
Text Solution
|