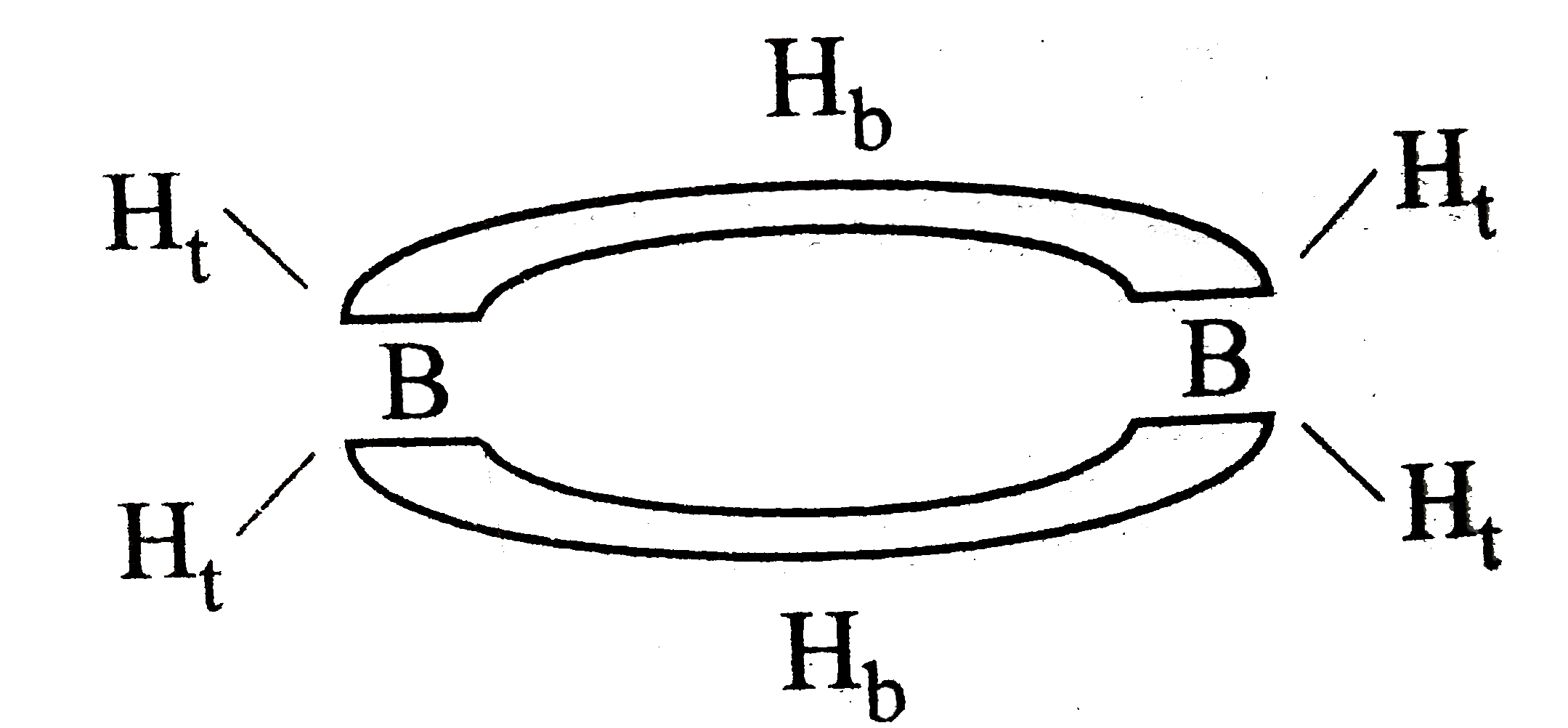
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise Molecular Orbitial Theory|42 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise Force Of Attraction|22 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise Valence Bond Theory (Vbt)|30 VideosATOMIC STRUCTURE
A2Z|Exercise Section D - Chapter End Test|30 VideosCHEMICAL EQUILIBRIUM
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Vsepr Theory And Hybridisation
- Which species has the maximum number of lone pair of electrons on the ...
Text Solution
|
- Among CIF(3), BF(3) and NH(3) molencules the one with non-planar geome...
Text Solution
|
- Specify the coordination geometry around and the hybridisation of N an...
Text Solution
|
- Which of the following molecules planer planar geometry?
Text Solution
|
- The two types of bonds present in B(2)H(6) are covalent and .
Text Solution
|
- Which has regular tetrahedral geometary ?
Text Solution
|
- Which of the following are isolectronic and iso-structural ? NO(3)^(...
Text Solution
|
- The percentage s-character of the hybrid orbitals in methane , ethene ...
Text Solution
|
- Among the compounds BF(3), NCI(3), H(2)S, SF(4) and BeCI(2)., identify...
Text Solution
|
- Total number of lone pair of electrons in XeOF4 is :
Text Solution
|
- Indicate the incorrect statement:
Text Solution
|
- Which iof the folowing molecule contains one pair of non-bonding elect...
Text Solution
|
- The hybridisation of the central atom will change when
Text Solution
|
- Which of the following is most stable
Text Solution
|
- The states of hybridisation of boron and oxygen atoms in boric acid (H...
Text Solution
|
- Sulphur reacts with chlorine in 1:2 ratio and forms X hydrolysis of X ...
Text Solution
|
- In XeF2, XeF4 and XeF6, the number of lone pair of electrons on Xe ar...
Text Solution
|
- The snecies having pyramidal shapes is
Text Solution
|
- The shapes of XeO(2)F(2) molecule is
Text Solution
|
- The pair of species having identical shape of both species :
Text Solution
|