A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MARVEL PUBLICATION-KINETIC THEORY OF GASES ,THERMODYNAMICS AND RADIATION-TEST YOUR GRASP - 9
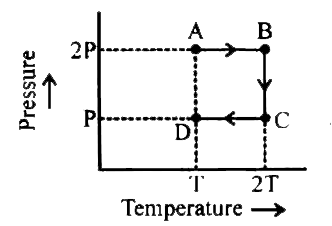
- An ideal monoatomic gas is taken through the thermodynamic states AtoB...
Text Solution
|
- The velocities of 4 molecules are 4m/s, 5m/s, 6m/s and 2m/s respective...
Text Solution
|
- Oxygen and hydrogen gas are at same temperature and pressure. And the ...
Text Solution
|
- The mean free path of nitrogen molecules at 27^(@)C is 3xx10^(-7)m//s....
Text Solution
|
- The equation of state for 15 gram of oxygen at pressure P, volume V an...
Text Solution
|
- The rms velocity of the molecules of a gas at temperature T is 1200 m/...
Text Solution
|
- If atmospheric pressure P=1xx10^(5)N//m^(2)andR=8J//"mole"//""^(@)K, t...
Text Solution
|
- At what temperature is the K.E. Of a gas molecules half that of its va...
Text Solution
|
- Each molecule of a gas has F degrees of freedom . The ratio (C(p))/(C(...
Text Solution
|
- Argon gas is heated at constant volume through 1^(@)C. The heat suppli...
Text Solution
|
- C(v) denotes the molar specific heat of a gas at constant volume and g...
Text Solution
|
- One mole of a monoatomic ideal gas is mixed with one mole of a diato...
Text Solution
|
- Which one of the following is not true about the following thermodynam...
Text Solution
|
- During an adiabatic process, the pressure of gas is found to be propor...
Text Solution
|
- The difference between the principal specific heats of a gas is 300J/k...
Text Solution
|
- When the temperature of 4 moles of a gas was increased from 80^(@)C to...
Text Solution
|
- 100g of water is heated from 30^@C to 50^@C. Ignoring the slight expan...
Text Solution
|
- The temperature of sink of Carnot engine is 27^(@)C. Efficiency of eng...
Text Solution
|
- In an ideal refrigerator, heat from inside at 280 K is transferred to ...
Text Solution
|
- Which one of the following statements is true in the case of radiation...
Text Solution
|
- A perfectly black sphere of diameter 0.2 m is in thermal equilibrium w...
Text Solution
|