A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
R SHARMA|Exercise Follow-up Test 4|15 VideosREDOX REACTIONS
R SHARMA|Exercise Follow-up Test 5|8 VideosREDOX REACTIONS
R SHARMA|Exercise Follow-up Test 2|5 VideosPURIFICATION AND CHARATERIZATION OF ORGANIC COMPOUNDS
R SHARMA|Exercise Archives|38 VideosSOME BASIC CONCEPTS OF CHEMISTRY
R SHARMA|Exercise Archives|26 Videos
Similar Questions
Explore conceptually related problems
R SHARMA-REDOX REACTIONS-Follow-up Test 3
- The oxidation nuber fo an atom in a given species (molecule ion, or fr...
Text Solution
|
- Oxidation is a process which involves .
Text Solution
|
- Reduction is a process wich involves (i) decrease in the oxidation ...
Text Solution
|
- Which of the following has zero oxidation number fro every atom ?
Text Solution
|
- The oxidation number of nitrogen in nitride ion is
Text Solution
|
- The sum of oxidation numbers fo all the atoms in the dichromate ion is...
Text Solution
|
- Fluorine can have an oxidation number of .
Text Solution
|
- The oxidation number of hyrogen is (i) 0 (ii) +1 (iii) -1 ...
Text Solution
|
- The oxidation number of N nitric acid molecule is .
Text Solution
|
- Which fo the follwing compounds fo oxygen has fractional oxdation numb...
Text Solution
|
- Oxidizing agents are spectes that
Text Solution
|
- Which fo the follwing can act as an oxidizing agent ?
Text Solution
|
- Which fo the following can function as a reducing agent ?
Text Solution
|
- Which of the following can funtion as an oxidizing as well as a reduci...
Text Solution
|
- Which of the noble gases exhibits the maximum number fo different oxid...
Text Solution
|
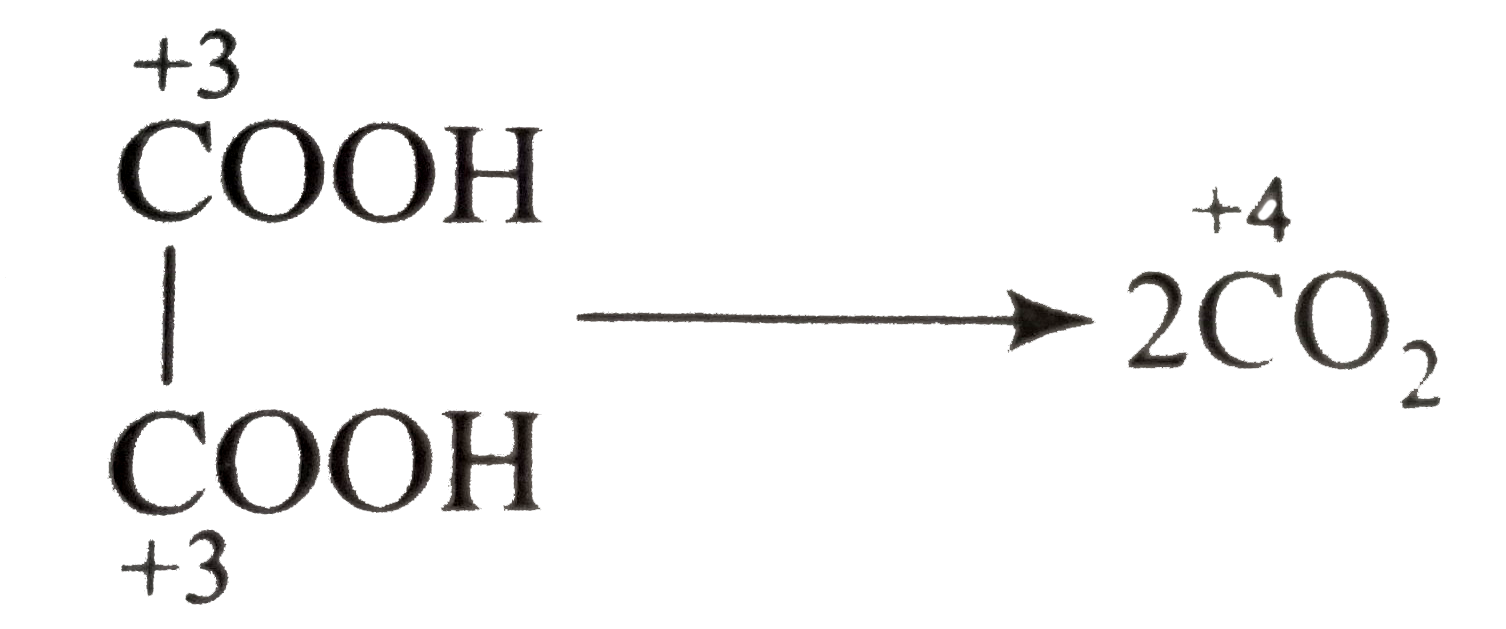 .
.