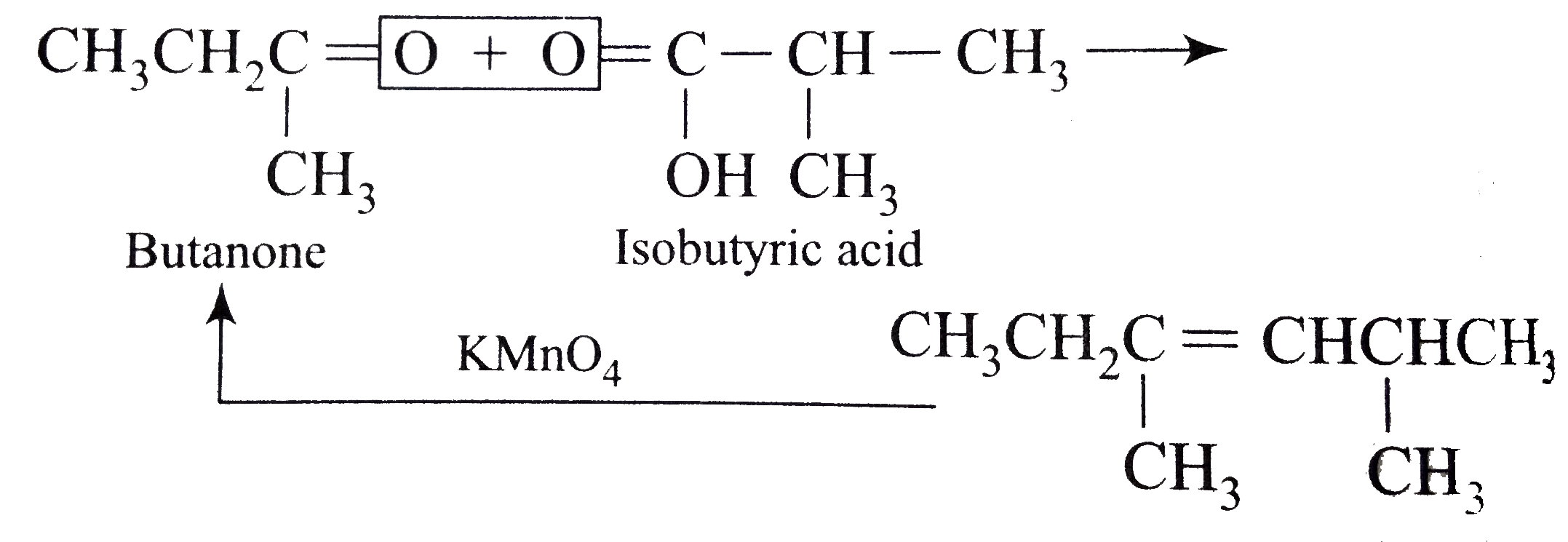
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-ALKENES-Follow-up-12
- Which of the following reagents can distinguish between propene and pr...
Text Solution
|
- Ethene reacts with cold dilute KMnO(4) to produce
Text Solution
|
- The olefin which on ozonolysis gives CH(3)CH(2)CHO and CH(3)CHO is
Text Solution
|
- But-2-"ene" underset(C Cl(4))overset(N-"Bromosuccinimide")rarr The p...
Text Solution
|
- An alkene on ozonolysis gives isobutyric acid only. The alkene is
Text Solution
|
- CH(3)CH=CH(2)underset(CH(2)Cl(2))overset(m-"Chloroperoxbenzoic acid")r...
Text Solution
|
- Identify the product P.
Text Solution
|
- A hydrocarbon C(8)H(16) on oxidation with a hot adidified solution of ...
Text Solution
|