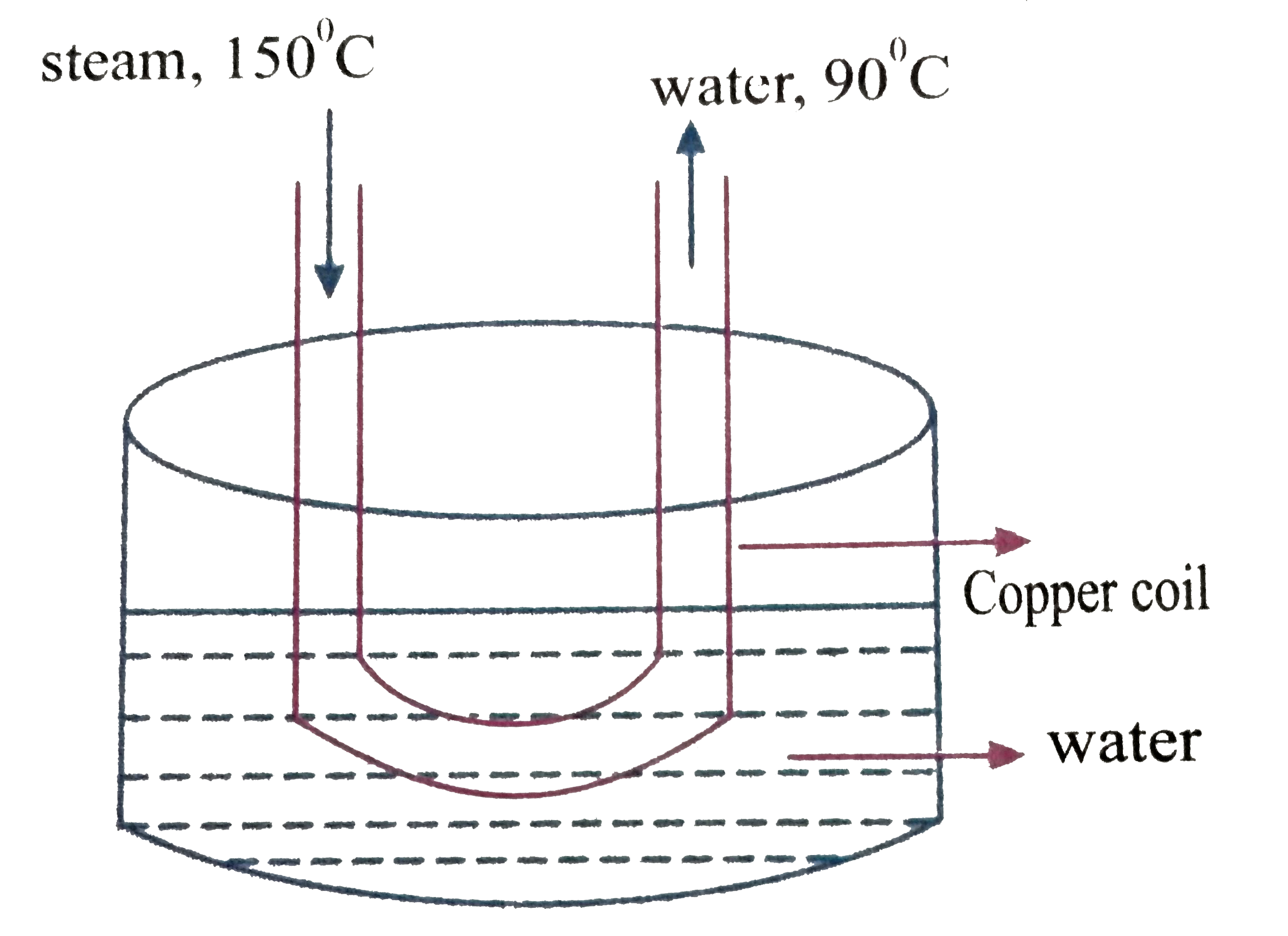
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-CALORIMETRY-Level-III (C.W)
- 30 kg ice at 0^@C and 20 g of steam at 100^@C are mixed. The compositi...
Text Solution
|
- 30 gms of water at 30^@C is in a beaker. Which of the following, when ...
Text Solution
|
- n' number of liquids of masses m,2m,3m,4m, ….. Having specific heats S...
Text Solution
|
- Steam is passed into a calorimeter with water having total thermal cap...
Text Solution
|
- 2kg of ice at 20^@C is mixed with 5kg of water at 20^@C in an insulati...
Text Solution
|
- A thermal insulated vessel contains some water at 0^(@)C. The vessel i...
Text Solution
|
- The specific heat of a substance varies with temperature t(.^(@)C) as ...
Text Solution
|
- In an industrial process 10 kg of water per hour is to be heated from ...
Text Solution
|
- A heater melts 0^@C ice in a bucket completely into water in 6 minute...
Text Solution
|
- Ice at 0^@C is added to 200 g of water initially at 70^@C in a vacuum ...
Text Solution
|
- A kettle with 2 litre water at 27^@C is heated by operating coil heate...
Text Solution
|