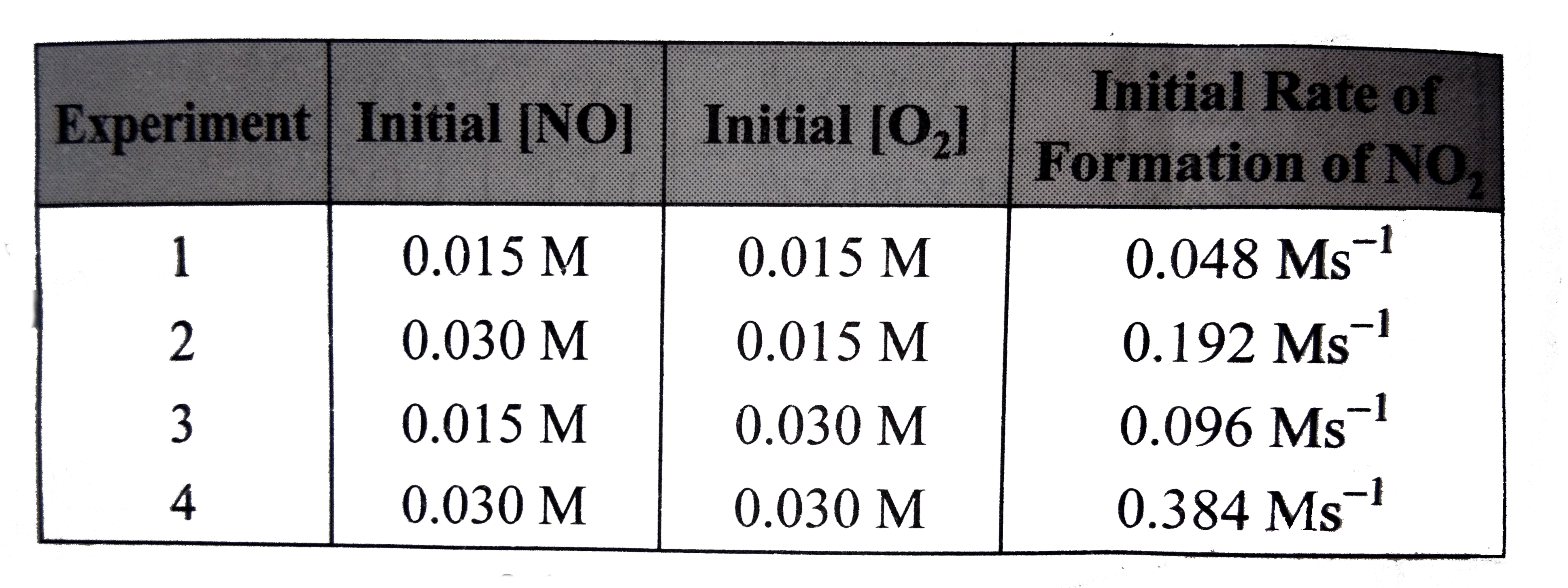
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-CHEMICAL KINETICS-Follow-up 2
- Which of the following is not a correct statement ?
Text Solution
|
- Which of the following illustrates the influence of surface area of a ...
Text Solution
|
- Which of the following is a very fast reaction ?
Text Solution
|
- Rate law is an expression relating the rate of a reaction to the
Text Solution
|
- The specific reaction rate (or rate constant) of a reaction depends up...
Text Solution
|
- The units of rate constant and rate of a reaction are idential for:
Text Solution
|
- Which of the following is not a correct statement ?
Text Solution
|
- The rate constant is unmerically the same for three reactions of first...
Text Solution
|
- 2N(2)O(5) rarr 4NO(2) + O(2) If (-d[N(2)O(5)])/(dt) = k(1)[N(2)O(5)]...
Text Solution
|
- The air oxidation of nitric oxide is one of the reactions that contri...
Text Solution
|