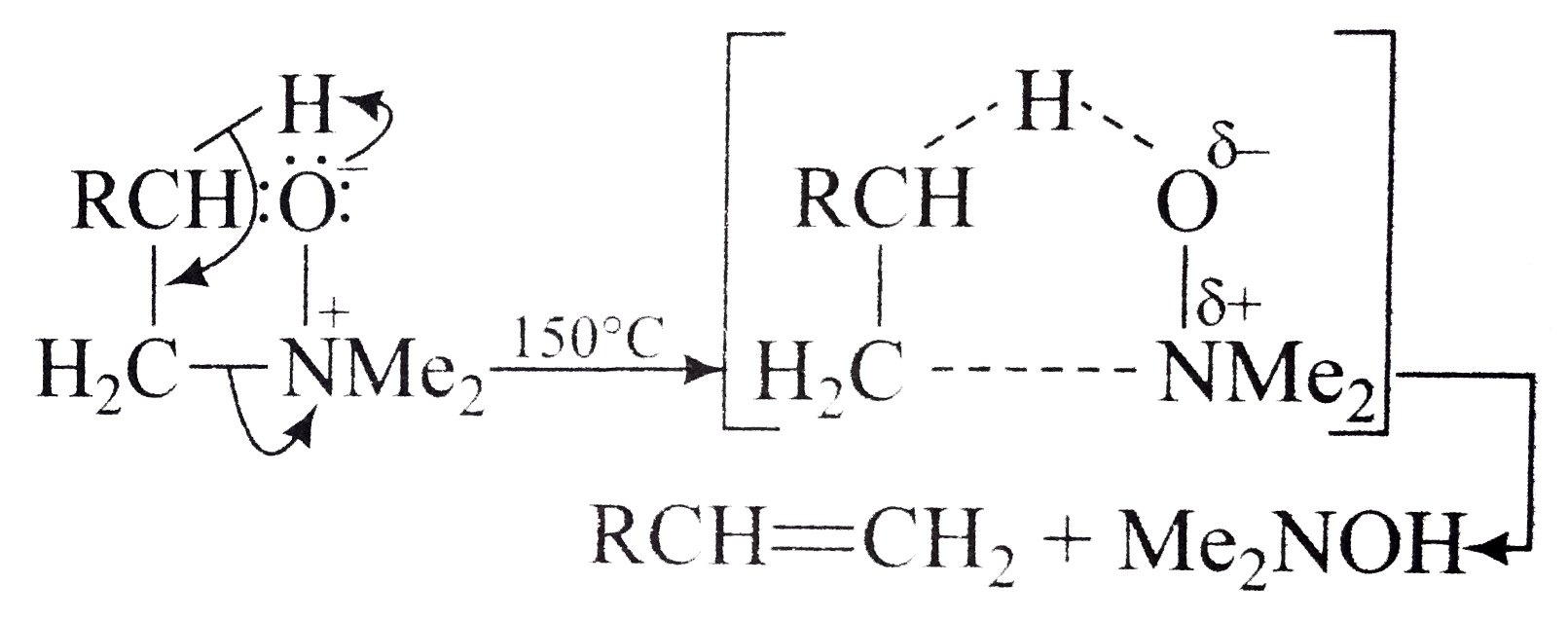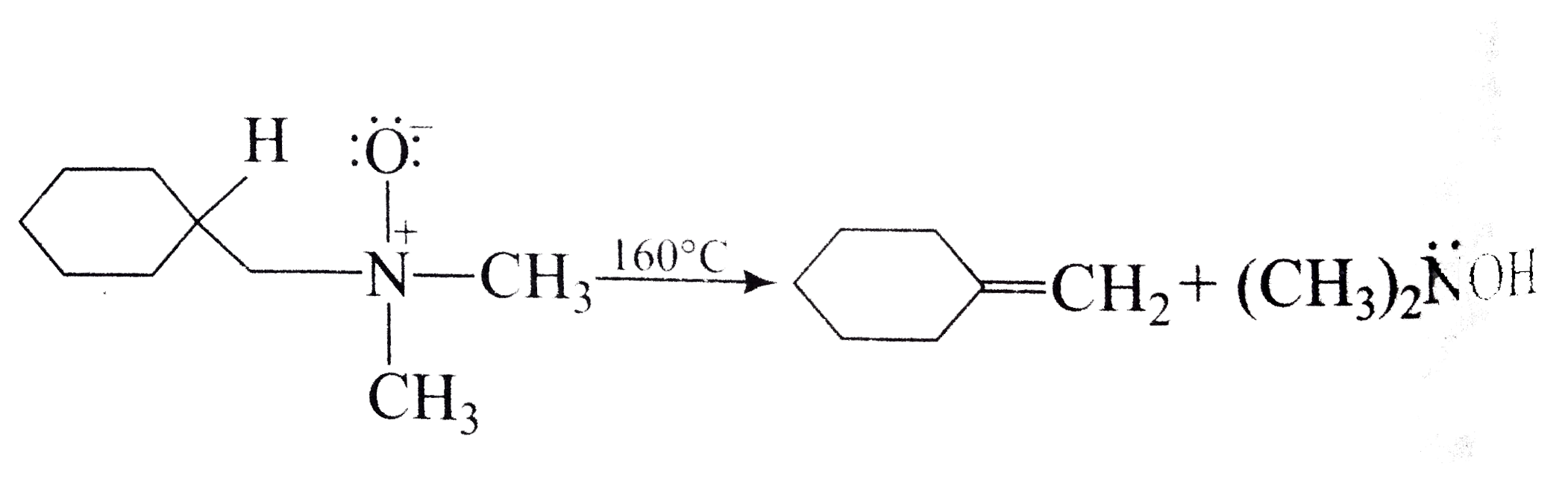A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-AMINES-Follow -up Test
- When refluxed with ethanolic carbon disulphide and solid potassium hyd...
Text Solution
|
- When diethylamine is oxidized by KMnO(4) the product is .
Text Solution
|
- Which of the following does not oxidise a tertiary amine to the amine ...
Text Solution
|
- Tertiary amine oxides undergo the elimination of a dialkyihydroxylamin...
Text Solution
|
- Aniline is oxidized by peroxytrifluoro acetic acid in methy1ene dichlo...
Text Solution
|
- Predict the product of the following reaction C(6)H(5)MgBr+CH(3)NH(2...
Text Solution
|
- Butan-1 amine reacts with nitrous acid at 0.^(@)C to form .
Text Solution
|
- Which one of the following compounds will produce a water insoluble ye...
Text Solution
|
- Which of the following amines undergo Liebermann s nitroso reaction ?
Text Solution
|
- The presence of a primary amine can be confirmed by its reaction with ...
Text Solution
|
- Which of the following is known as Hinsberg reagent ?
Text Solution
|
- Consider the following sequence of reactions NO(2) The end product ...
Text Solution
|
- Consider the reaction The name of the reactions and the intermediate v...
Text Solution
|
- Consider the following sequence of reactions .
Text Solution
|
- In the chemical reactions the compounds (A) and (B) are .
Text Solution
|
- Predict the final product of the following sequence of reactions
Text Solution
|
- Benzenediazonium chloride does not react with .
Text Solution
|
- Which of the following conditions is most satisfactory for diazo coupl...
Text Solution
|
- Methy1 orange an acid-base indicator is prepared by diazo coupling rea...
Text Solution
|
- Among the diazonium ions the order of reactivity towards diazocoupling...
Text Solution
|