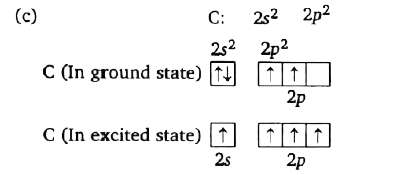
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING (BASIC)
VK JAISWAL|Exercise Level 3 (Passive 3)|6 VideosCHEMICAL BONDING (BASIC)
VK JAISWAL|Exercise Level 3 (Passive 4)|6 VideosCHEMICAL BONDING (BASIC)
VK JAISWAL|Exercise SUBJECTIVE PROBLEMS|54 VideosCHEMICAL BONDING (ADVANCED)
VK JAISWAL|Exercise SUBJECTIVE PROBLEMS|72 VideosCO-ORDINATION COMPOUNDS
VK JAISWAL|Exercise ASSERTION-REASON TYPE QUESTIONS|26 Videos
Similar Questions
Explore conceptually related problems
VK JAISWAL-CHEMICAL BONDING (BASIC)-Level 2
- Which of the following compound has maximum "C-C" single bond length ?
Text Solution
|
- If two different non-axial d-orbitals having 'xz' nodal plane form pi-...
Text Solution
|
- Assuming pure 2s and 2p orbitals of carbon are used in forming CH(4) m...
Text Solution
|
- The strength of bonds by 2s -2s, 2p2p and 2p-2s overlap has the order
Text Solution
|
- Which of the following statement is not correct for sigma and pi- bond...
Text Solution
|
- Assuming the bond direction to the z-axis, which of the overlapping of...
Text Solution
|
- Which of the following orbital can not form pi as well as delta-Bond ?
Text Solution
|
- Incorrect statement is :
Text Solution
|
- Which of the following set contains species having same angle around t...
Text Solution
|
- Which of the following compound has the smallest (X-A-X) bond angle in...
Text Solution
|
- The incorrect order of boiling point is :
Text Solution
|
- Iodine molecules are held in the solid lattice by
Text Solution
|
- Carbon dioxide is a gas but silica is a solid because:
Text Solution
|
- Choose the correct code of characteristics for the given order of hybr...
Text Solution
|
- Which is correct statement ? As the s-character of a hybrid orbital ...
Text Solution
|
- Which of the following is incorrectly matched ?
Text Solution
|
- The ionic bond X^(+)Y^(-) are formed when : (I) electron affinity of...
Text Solution
|
- In the Born-Haber cycle for the formation of solid common salt (NaCl),...
Text Solution
|
- Species having maximum 'Cl-O' bond order is :
Text Solution
|
- Which of the following species contains minimum number of atoms in XY ...
Text Solution
|