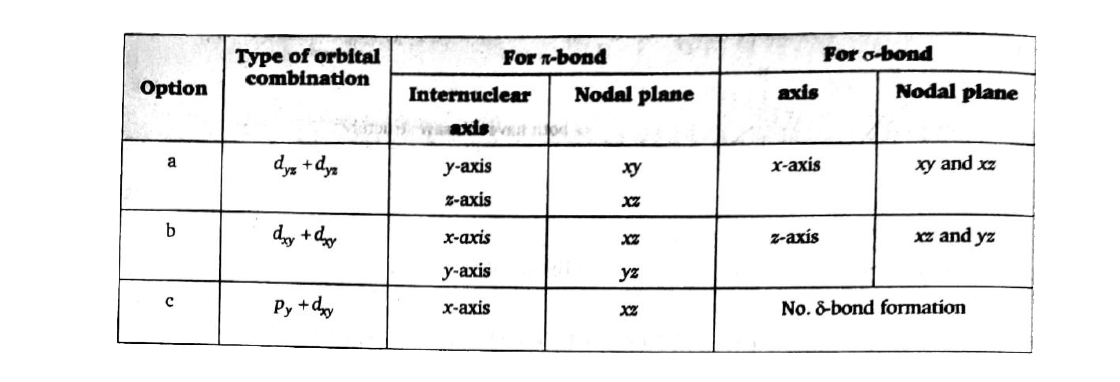
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING (BASIC)
VK JAISWAL|Exercise Level 3 (Passive 7)|6 VideosCHEMICAL BONDING (BASIC)
VK JAISWAL|Exercise Level 3 (Passive 8)|4 VideosCHEMICAL BONDING (BASIC)
VK JAISWAL|Exercise Level 3 (Passive 1)|6 VideosCHEMICAL BONDING (ADVANCED)
VK JAISWAL|Exercise SUBJECTIVE PROBLEMS|72 VideosCO-ORDINATION COMPOUNDS
VK JAISWAL|Exercise ASSERTION-REASON TYPE QUESTIONS|26 Videos
Similar Questions
Explore conceptually related problems
VK JAISWAL-CHEMICAL BONDING (BASIC)-Level 3 (Passive 2)
- According to VBT any covalent bond will be formed by overlapping of at...
Text Solution
|
- According to VBT any covalent bond will be formed by overlapping of at...
Text Solution
|
- According to VBT any covalent bond will be formed by overlapping of at...
Text Solution
|
- According to VBT any covalent bond will be formed by overlapping of at...
Text Solution
|
- According to VBT any covalent bond will be formed by overlapping of at...
Text Solution
|
- According to VBT any covalent bond will be formed by overlapping of at...
Text Solution
|