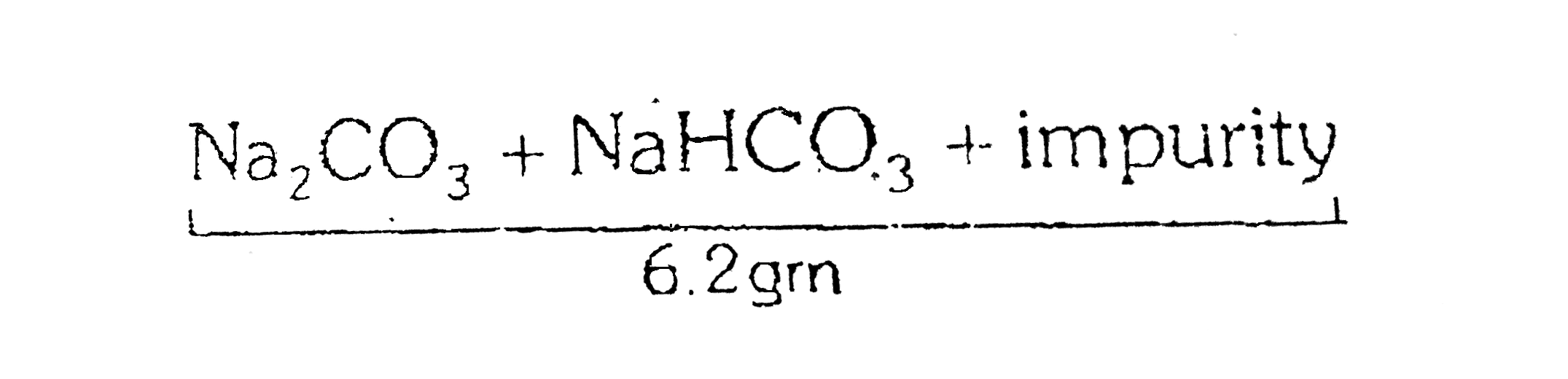Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-MOLE CONCEPT-Exercise - 04 [A]
- A plant virus is found to consist of uniform cylindrical particle of 1...
Text Solution
|
- Density of a gas relative t air is 1.17. Find the mol. Mass of the ga...
Text Solution
|
- One type of artificial diamond (commonly called YAG for yttrium alum...
Text Solution
|
- A chemical commonly called "dioxin" has been very much in the news in ...
Text Solution
|
- A chemist wants to prepare diborane by the reaction BF(3) rarr 6 L...
Text Solution
|
- One gram of an alloy of aluminium and magnesium when heated with exces...
Text Solution
|
- A 10.0 g sample of a mixture of CaCl2 and NaCl is treated to precipita...
Text Solution
|
- By the reaction of carbon and oxygen, a mixture of CO and CO(2) is obt...
Text Solution
|
- Cadverine molecule has 58.77% C, 13.81% H and 27% N by mass Find Empir...
Text Solution
|
- Given the following empirical formula and molecular weight, compute th...
Text Solution
|
- What is the percentage of nitrogen in an organic compound 0.14 g of wh...
Text Solution
|
- Calculate the molarity of the following solution: (a) 4g of caustic ...
Text Solution
|
- A mixture of ethanol and water contains 54% water by mass. Calculate t...
Text Solution
|
- Ten millilitre of a mixture of CO,CH4, and N2 exploded with an excess ...
Text Solution
|
- When 100 ml of O2-O3 mixture was passed through turpentine oil, there ...
Text Solution
|
- Nitric acid can be produced from ammonia in three step process. {:(...
Text Solution
|
- A mineral consists of an equimolar mixture of the carbonates of two bi...
Text Solution
|
- 6.2 g of a sample containing NaHCO(3), NaHCO(3) and non -volatiale in...
Text Solution
|
- An unknown metal reacts with excess chlorine to give the metal chlorid...
Text Solution
|
- Cholrine gas can be produced by the reaction of HCl (aq) with MnO(2) (...
Text Solution
|