A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALLEN-ELECTROCHEMISTRY-MISCELLANEOUS SOLVED EXAMPLES
- Given standard electrode potentials K^(o+)|K=-2.93V, Ag^(o+)|Ag=0.80...
Text Solution
|
- A cell is prepared by dipping a copper rod in 1 MCuSO(4) solution and ...
Text Solution
|
- Predict whether the following reaction can occur under standard condit...
Text Solution
|
- Given that, Co^(3+) +e^(-)rarr Co^(2+) E^(@) = +1.82 V 2H(2)O rarr O...
Text Solution
|
- The measured e.m.f. at 25^(@)C for the cell reaction, Zn(s) +XCu^(2+...
Text Solution
|
- Calculate DeltaG^(@) for the reaction : Cu^(2+)(aq) +Fe(s) hArr Fe^(2+...
Text Solution
|
- A solution of copper (II) sulphate is electrolysed between copper elec...
Text Solution
|
- Calculate the equilibrium constant for the reaction at 298K. Zn(s) +...
Text Solution
|
- Calculate the cell e.m.f. and DeltaG for the cell reaction at 298K for...
Text Solution
|
- The emf of a cell corresponding to the reaction Zn +2H^(+)(aq) rarr ...
Text Solution
|
- Find the standard electrode potential of MnO(4)^(c-)|MnO(2). The stand...
Text Solution
|
- The half cell potential of a half-cell A^(x+), A^((x+n)+)|Pt were foun...
Text Solution
|
- Consider the given data. {:(,"Half-cell reaction","Standard reductio...
Text Solution
|
- Specific conductance of 10^(-4)M n-Butyric acid aqueous solution is 1....
Text Solution
|
- How much time is required for complete decomposition of two moles of w...
Text Solution
|
- The cell Pt|H(2)(g) (1atm)|H^(+), pH = x || Normal calomal electrode h...
Text Solution
|
- The specific conductivity of a saturated AgCI solution if found to be ...
Text Solution
|
- Consider the following standard reduction potentials:- {:(Fe^(2+)+2e...
Text Solution
|
- Find ^^(m)^(oo) ("in" Omega^(-1) cm^(2) mol^(-1)) for strong electroyt...
Text Solution
|
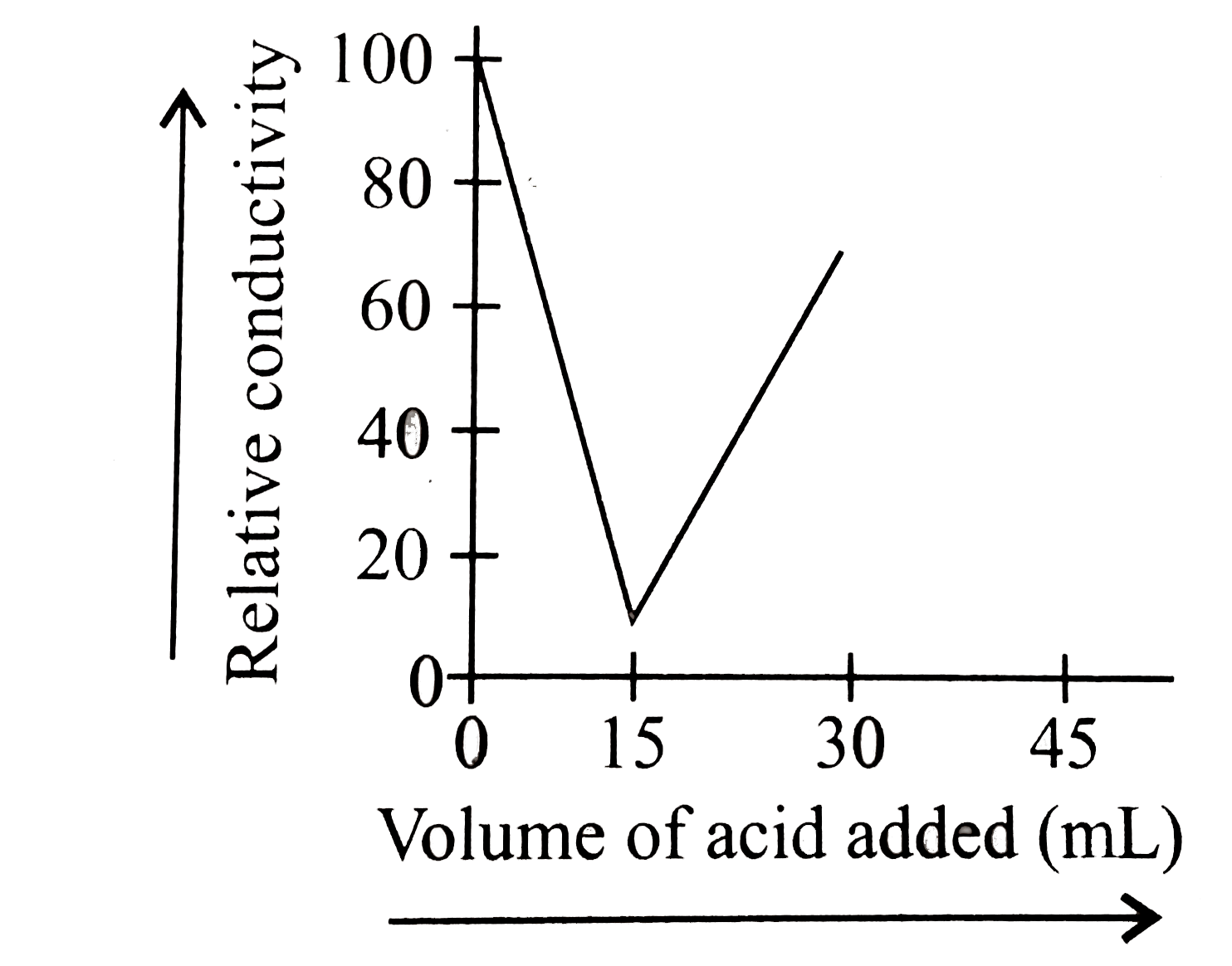
- 20 Ml of KOH solution was titrated with 0.20 M H(2)SO(4) solution in a...
Text Solution
|