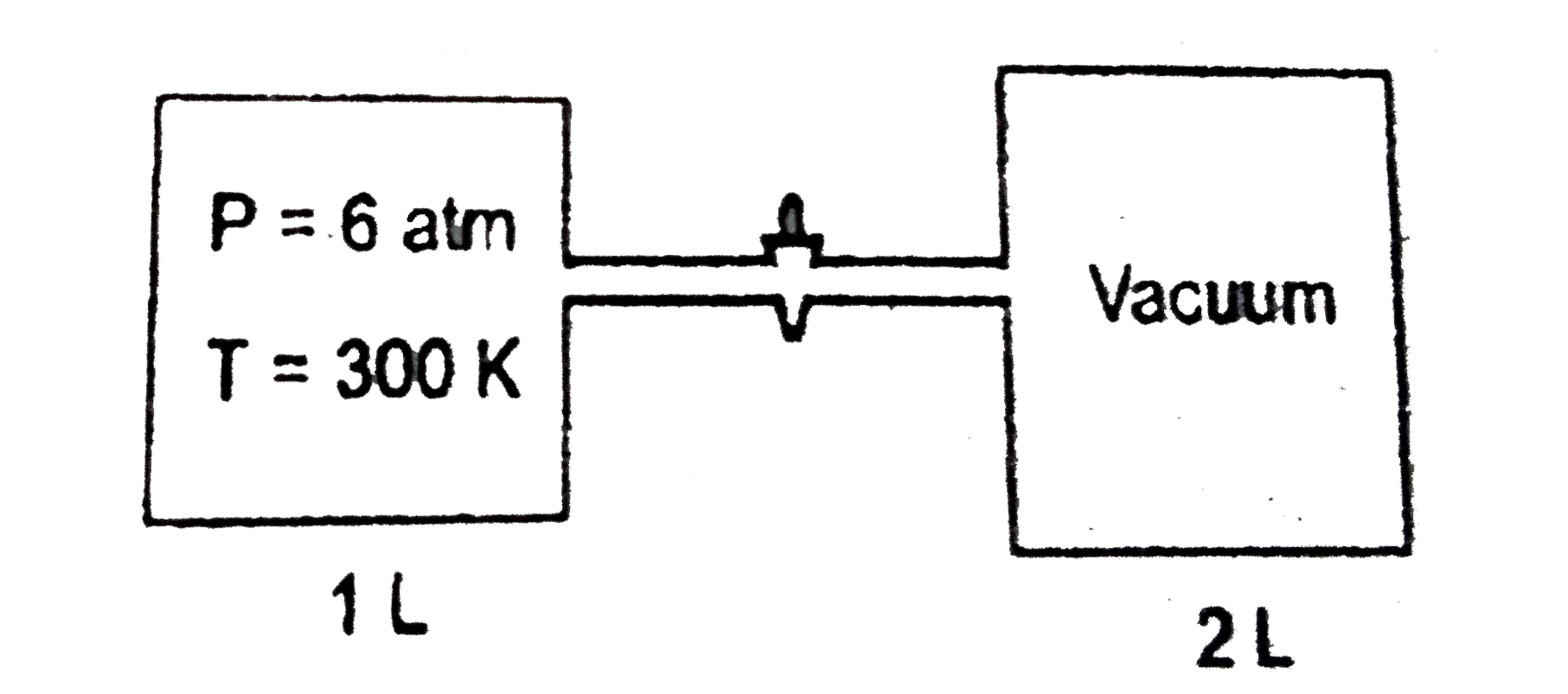
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-TEST PAPERS-PART - III CHEMISTRY
- When a sample of N(2) gas is passed through a liquid (N(2) is insolubl...
Text Solution
|
- It is known fact that gases expand to fill all available volume and th...
Text Solution
|
- It is known fact that gases expand to fill all available volume and th...
Text Solution
|
- Adenine is a substituted form of a heterocyclic nitrogenous base Purin...
Text Solution
|
- Adenine is a substituted form of a heterocyclic nitrogenous base Purin...
Text Solution
|
- Valence shell electron pair repusin theory (VSEPR) can be used to perd...
Text Solution
|
- Valence shell electron pair repusin theory (VSEPR) can be used to perd...
Text Solution
|
- HCN and HNC molcules are formed by the same atoms. Which of the foll...
Text Solution
|
- HCN and HNC molcules are formed by the same atoms. What is correct a...
Text Solution
|
- The energy levels in a certain single electron species are all 800% hi...
Text Solution
|
- The slope of 'P' v/s "T" plot at constant volume for a fixed amount of...
Text Solution
|
- Consider the reaction C(6)H(12)O(6) + 12H(2)SO(4) rarr 6CO(2) + 12SO...
Text Solution
|
- Calculate the molecular weight of a gas X which diffuses four times as...
Text Solution
|
- Total number of electrons having n + l = 4 in V(23) atom in its ground...
Text Solution
|
- How many of the following elements show +2 as their most stable oxidat...
Text Solution
|
- How many of following anions have 1.5 bond order ? IO(4^(-)), CH(3)C...
Text Solution
|
- How many of the following have sp^(3) hydridisation around the central...
Text Solution
|
- Cl-O band order in perchlorate ion is M and number of resonating struc...
Text Solution
|
- Write the sum of lone pairs of electron on central atom of SF(4), CF(4...
Text Solution
|
- A hydrogen like atom in ground state absorbs n photon having the sa...
Text Solution
|