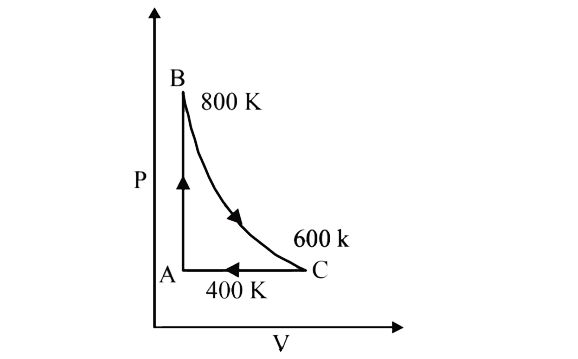
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
KTG & THERMODYNAMICS
RESONANCE|Exercise Advancel Level Problems|1 VideosKTG & THERMODYNAMICS
RESONANCE|Exercise SUBJECTIVE QUESTIONS|27 VideosKTG & THERMODYNAMICS
RESONANCE|Exercise PART - I|18 VideosKINETIC THEORY OF GASES AND THERMODYNAMICS
RESONANCE|Exercise Exercise|64 VideosMAGNETIC FIELD AND FORCES
RESONANCE|Exercise Exercise|65 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-KTG & THERMODYNAMICS-PART - II
- If CP and CV denote the specific heats of nitrogen per unit mass at co...
Text Solution
|
- When a system is taken from state i to state f along the path iaf, it ...
Text Solution
|
- An insulated container of gas has two chambers separated by an insulat...
Text Solution
|
- Two moles of helium gas are taken over the cycle ABCDA, as shown in th...
Text Solution
|
- Two moles of helium gas are taken over the cycle ABCDA, as shown in th...
Text Solution
|
- Two moles of helium gas are taken over the cycle ABCDA, as shown in th...
Text Solution
|
- One kg of a diatomic gas is at pressure of 8xx10^4N//m^2. The density ...
Text Solution
|
- A diatomic ideal gas is used in a Carnot engine as the working substan...
Text Solution
|
- 100g of water is heated from 30^@C to 50^@C. Ignoring the slight expan...
Text Solution
|
- A Carnot engine operating between temperature T1 and T2 has efficiency...
Text Solution
|
- Three perfect gases at absolute temperature T(1), T(2) and T(3) are mi...
Text Solution
|
- A thermally insulated vessel contains an ideal gas of molecular mass M...
Text Solution
|
- A container with insulating walls is divided into two equal parts by a...
Text Solution
|
- Helium gas goes through a cycle ABCDA (consisting of two isochoric and...
Text Solution
|
- The above p-v diagram represents the thermodynamic cycle of an engine,...
Text Solution
|
- One mole of a diatomic ideal gas undergoes a cyclic process ABC as sho...
Text Solution
|
- An open glass tube is immersed in mercury in such a way that a lenth o...
Text Solution
|
- Consider a spherical shell of radius R at temperature T. The black bod...
Text Solution
|
- A solid body of constant heat capacity 1J//^@C is being heated by keep...
Text Solution
|
- Consider an ideal gas confined in an isolated closed chamber. As the g...
Text Solution
|