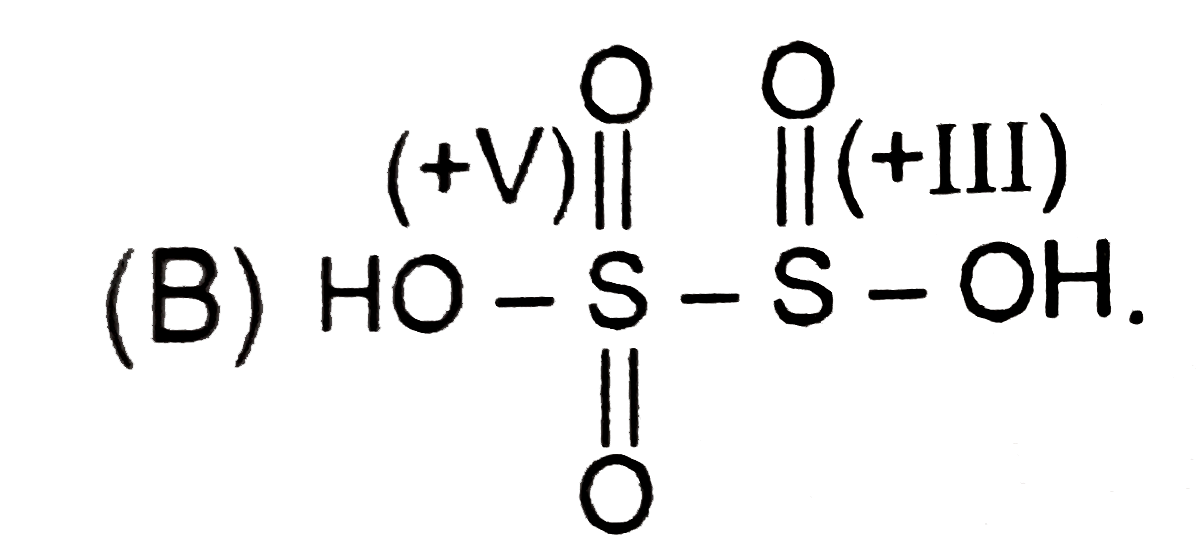A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
P BLOCK ELEMENTS
RESONANCE|Exercise ALP EX #3 OBJECTIVE|1 VideosP BLOCK ELEMENTS
RESONANCE|Exercise ALP PART 1 SUBJECTIVE|3 VideosP BLOCK ELEMENTS
RESONANCE|Exercise PART -III : Match the column|2 VideosNUCLEAR CHEMISTRY
RESONANCE|Exercise STAGE-II|1 VideosP-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE|Exercise PART - III : OLYMPIAD PROBLEMS (PREVIOUS YEARS) STAGE - V (INTERNATIONAL CHEMISTRY OLYMPIAD (IChO)) Problem 3|8 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-P BLOCK ELEMENTS-ALP PART IV : Comprehension
- Nitrogen forms the largest number of oxides as it is capable of formin...
Text Solution
|
- Nitrogen forms the largest number of oxides as it is capable of formin...
Text Solution
|
- An inorganic iodide (A) on heating with a solution of KOH gives a gas ...
Text Solution
|
- An inorganic iodide (A) on heating with a solution of KOH gives a gas ...
Text Solution
|
- An inorganic iodide (A) on heating with a solution of KOH gives a gas ...
Text Solution
|
- Oxygen differs from the other elements of the group. Compounds of oxyg...
Text Solution
|
- Oxygen differs from the other elements of the group. Compounds of oxyg...
Text Solution
|
- The property of hydrides of p-block elements mostly depends on : (i)...
Text Solution
|
- The property of hydrides of p-block elements mostly depends on : (i)...
Text Solution
|
- The property of hydrides of p-block elements mostly depends on : (i)...
Text Solution
|