Text Solution
Verified by Experts
The correct Answer is:
ARIHANT-FIRST LAW OF THERMODYNAMICS-Level 3
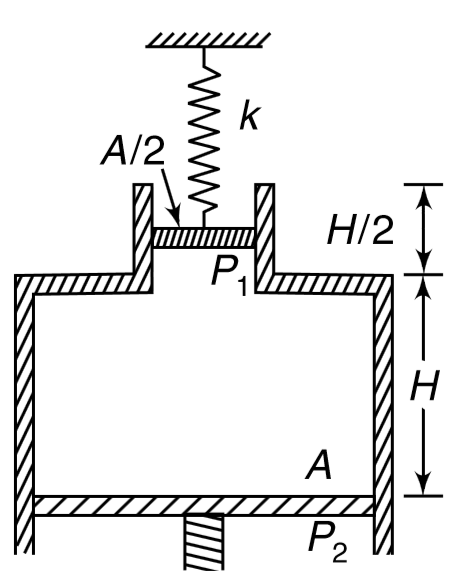
- An adiabatic cylinder of cross section A is fitted with a mass less co...
Text Solution
|
- One mole of an ideal gas is expanded from the state A(P(0), V(0)) to f...
Text Solution
|
- An ideal gas, in initial state 1(P(1), V(1), T(1)) is cooled to a stat...
Text Solution
|
- In ideal gas is enclosed in an adiabatic container having cross sectio...
Text Solution
|
- An insulated cylinder is divided into three parts A, B and C. Pistons ...
Text Solution
|
- An adiabatic cylinder has length 2 L and cross sectional area A. A fre...
Text Solution
|