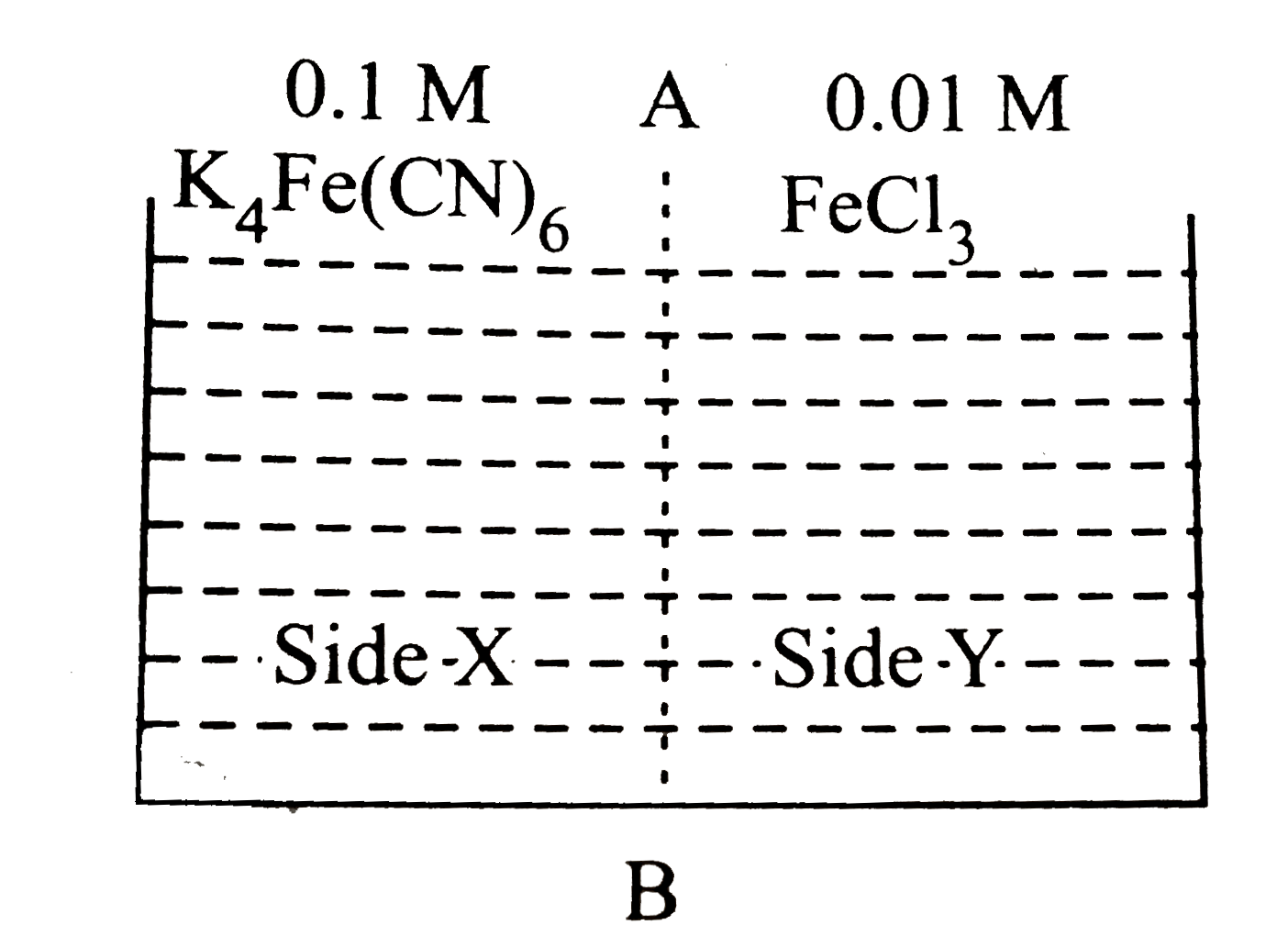A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLUTIONS
RESONANCE|Exercise EXERCISE-2(PART-4)|4 VideosSOLUTIONS
RESONANCE|Exercise EXERCISE-3(PART-1)|14 VideosSOLUTIONS
RESONANCE|Exercise EXERCISE-2(PART-1)|25 VideosSOLUTION AND COLLIGATIVE PROPERTIES
RESONANCE|Exercise PHYSICAL CHEMITRY (SOLUTION & COLLIGATIVE PROPERTIES)|52 VideosSTEREOISOMERISM
RESONANCE|Exercise EXERCISE (PART III : PRACTICE TEST-2 (IIT-JEE (ADVANCED PATTERN))|22 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-SOLUTIONS-EXERCISE-2(PART-2)
- The degree of dissociation of an electrolyte is alpha and its van't Ho...
Text Solution
|
- Barium ion , CN^(-) and Co^(2+) form an ionic complex . If that comple...
Text Solution
|
- The relative decreases in the vapour pressure of an aqueous solution c...
Text Solution
|
- In the following aqueous solutions (A) 1m surcose (B) 1m potassium f...
Text Solution
|
- Dry air is slowly passed through three solutions of different concentr...
Text Solution
|
- A pressure cooker reduces cooking time because
Text Solution
|
- A solute 'S' undergoe a reversibel trimerization when dissolved in a c...
Text Solution
|
- When only a little quantity ofHgCl(2)is added to excess Kl(aq) to obta...
Text Solution
|
- The freezing point of of aqueous solution that contains 3% urea. 7.45%...
Text Solution
|
- x mole of KCl and y mole of BaCl(2) are both dissolved in 1kg of water...
Text Solution
|
- For a solution of 0.849g of mercurous chloride in 50g of HgCl(2)(l) th...
Text Solution
|
- At a constant temperature , DeltaS will be maximum for which of the fo...
Text Solution
|
- FeCl(3) on reaction with K(4)[Fe(CN)(6)] in aqueous solution gives blu...
Text Solution
|
- Two beakers, one contaning 20ml of a 0.05M aqueous solution of a non v...
Text Solution
|
- The osmotic pressure of blood is 7.40 atm at 27^(@) C.The number of mo...
Text Solution
|
- Which of the following is/are correct for an ideal binary solution of ...
Text Solution
|
- The diagram given below represents boiling point composition diagram o...
Text Solution
|
- According to Henry's law, the partial pressure of gas (p'(g)) is direc...
Text Solution
|
- For the given electrolyte A(x)B(y). The degree of dissociation 'alpha'...
Text Solution
|
- A graph plotted between (P)/(d) vs d (where p is osmotic pressure of s...
Text Solution
|