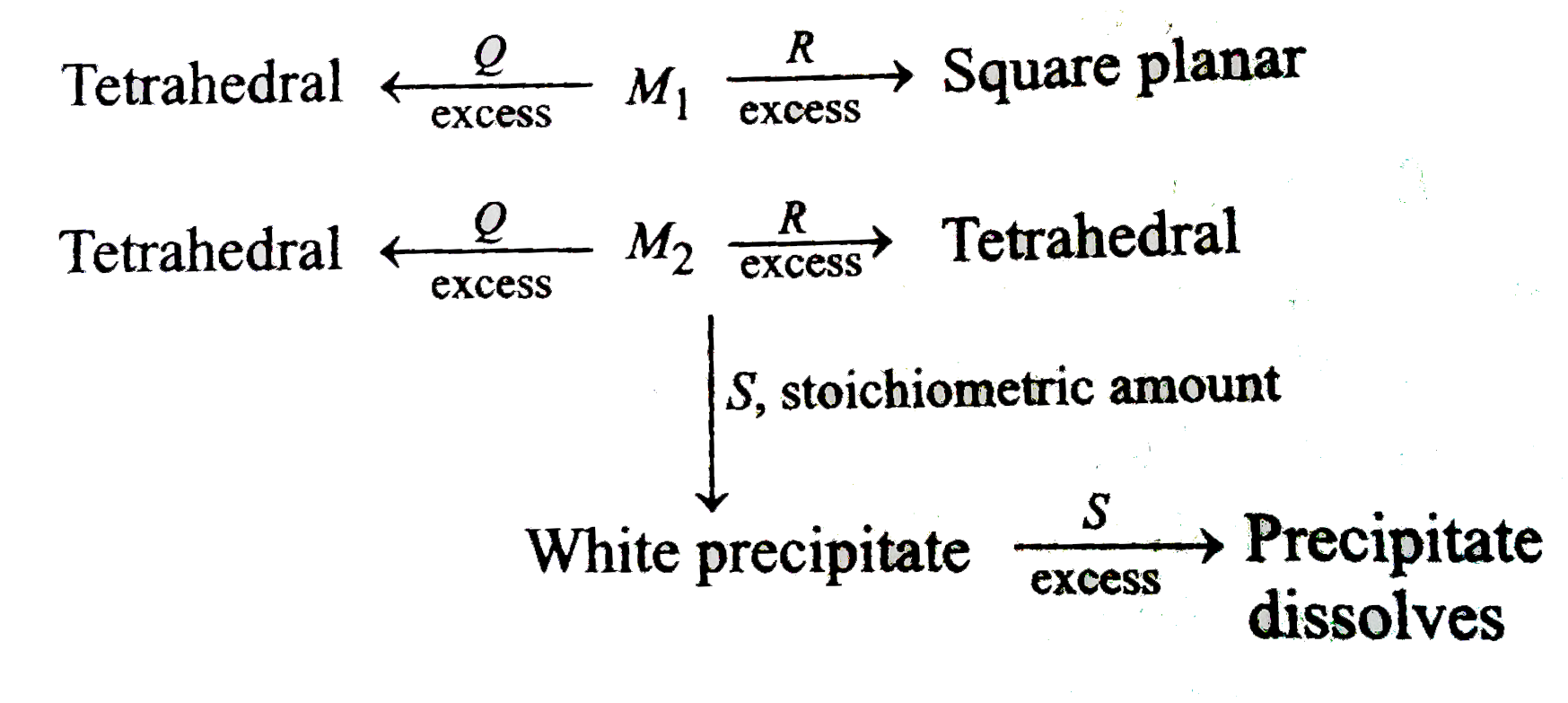A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
QUALITATIVE ANALYSIS
IIT-JEE PREVIOUS YEAR (CHEMISTRY)|Exercise Fill in the blanks|1 VideosQUALITATIVE ANALYSIS
IIT-JEE PREVIOUS YEAR (CHEMISTRY)|Exercise True/False|1 VideosQUALITATIVE ANALYSIS
IIT-JEE PREVIOUS YEAR (CHEMISTRY)|Exercise Objective Question I|3 VideosPERIODIC CLASSIFICATION AND PERIODIC PROPERTIES
IIT-JEE PREVIOUS YEAR (CHEMISTRY)|Exercise COMPREHENSION_TYPE|3 VideosS BLOCK ELEMENTS
IIT-JEE PREVIOUS YEAR (CHEMISTRY)|Exercise True/False|1 Videos
Similar Questions
Explore conceptually related problems
IIT-JEE PREVIOUS YEAR (CHEMISTRY)-QUALITATIVE ANALYSIS-Passage Based Questions
- An aqueous solution of metal ion M(1) reacts separately with reagents ...
Text Solution
|
- An aqueous solution of metal ion M(1) reacts separately with reagents ...
Text Solution
|
- When a metal rod M is dipped into an aqueous colourless concentrated s...
Text Solution
|
- p-amino-N, N-dimethyl laniline is added to a strongly acidci solution ...
Text Solution
|
- p-amino-N, N-dimethyl laniline is added to a strongly acidci solution ...
Text Solution
|
- p-amino-N, N-dimethyl laniline is added to a strongly acidci solution ...
Text Solution
|