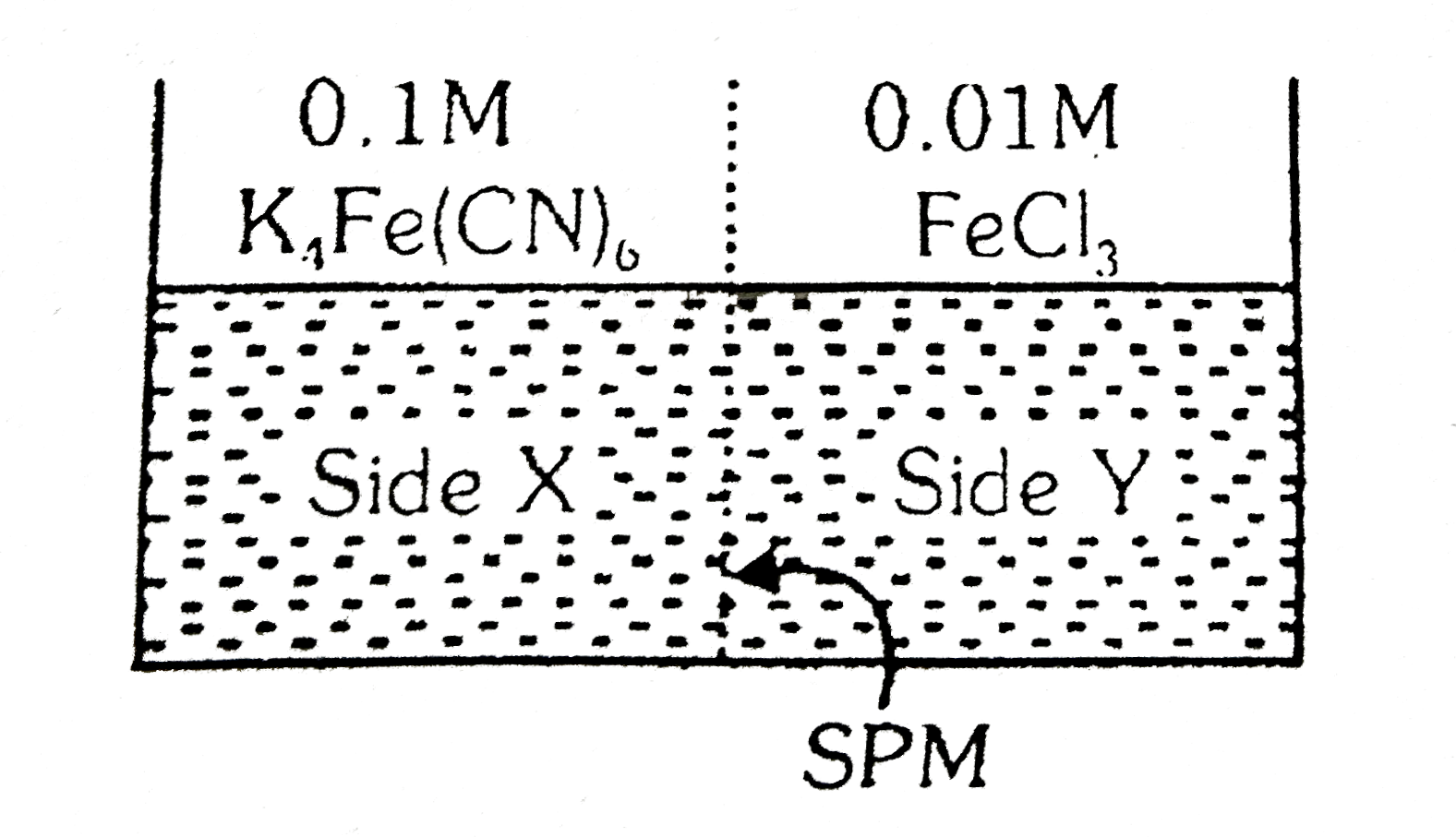A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-SOLUTIONS-Exercise 1
- The Van't Hoff factor for a dillute aqueous solution of glucose is
Text Solution
|
- The correct relationship between the boiling point f very dilute solut...
Text Solution
|
- The vapour pressure of a solution of a non-volatile electrolyte B in a...
Text Solution
|
- At a given temperature , total vapour pressure (in Torr) of a mixture ...
Text Solution
|
- Assuming each salt to be 90% dissociated which of the following will h...
Text Solution
|
- The vapour pressure of a solvent decreased by 10 mm of Hg when a non-v...
Text Solution
|
- Elevation of boiling point of 1 molar aqueous glucose solution ("densi...
Text Solution
|
- What will be the molecular weight of "CaCl"(2) determined in its aq. S...
Text Solution
|
- 1.0 molal aqueous solution of an electrolyte "A"(2)"B"(3) is 60% ionis...
Text Solution
|
- Which of the following plots represents an ideal binary mixture?
Text Solution
|
- Two liquid A & B form an ideal solution. What is the vapour pressure o...
Text Solution
|
- Which of the following represents correcty the changes in thermodynami...
Text Solution
|
- FeCI(3) on reaction with K(4)[Fe(CN)(6)] in aqueous solution gives blu...
Text Solution
|
- A liquid mixture ohaving composition corresponding to point Z in the f...
Text Solution
|
- The vapour pressure of a saturated solution of sparingly soluble salt ...
Text Solution
|
- At 300K, the vapour pressure of an ideal solution containing 3 mole of...
Text Solution
|
- The following graph represents variation of boiling point with composi...
Text Solution
|
- The freezing point depression of a 0.1 M aq. Solution of weak acid (HX...
Text Solution
|
- The vapour pressure of an aqueous solution is found to be 750 torr at...
Text Solution
|
- The following graph represents variation of vapour pressre with compos...
Text Solution
|