A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-SOLUTIONS-Exercise 2
- 1 mole of liquid A and 9 moles of liquid B are mixed to form a solutio...
Text Solution
|
- 1 mole of liquid A and 9 moles of liquid B are mixed to form a solutio...
Text Solution
|
- 1 mole of liquid A and 9 moles of liquid B are mixed to form a solutio...
Text Solution
|
- An isotope of hydrogen atom is represented as X which follows Bohr'r m...
Text Solution
|
- An isotope of hydrogen atom is represented as X which follows Bohr's ...
Text Solution
|
- An isotope of hydrogen atom is represented as X which follows Bohr'r m...
Text Solution
|
- These questions consists of two statements each, printed as Statement-...
Text Solution
|
- These questions consists of two statements each, printed as Statement-...
Text Solution
|
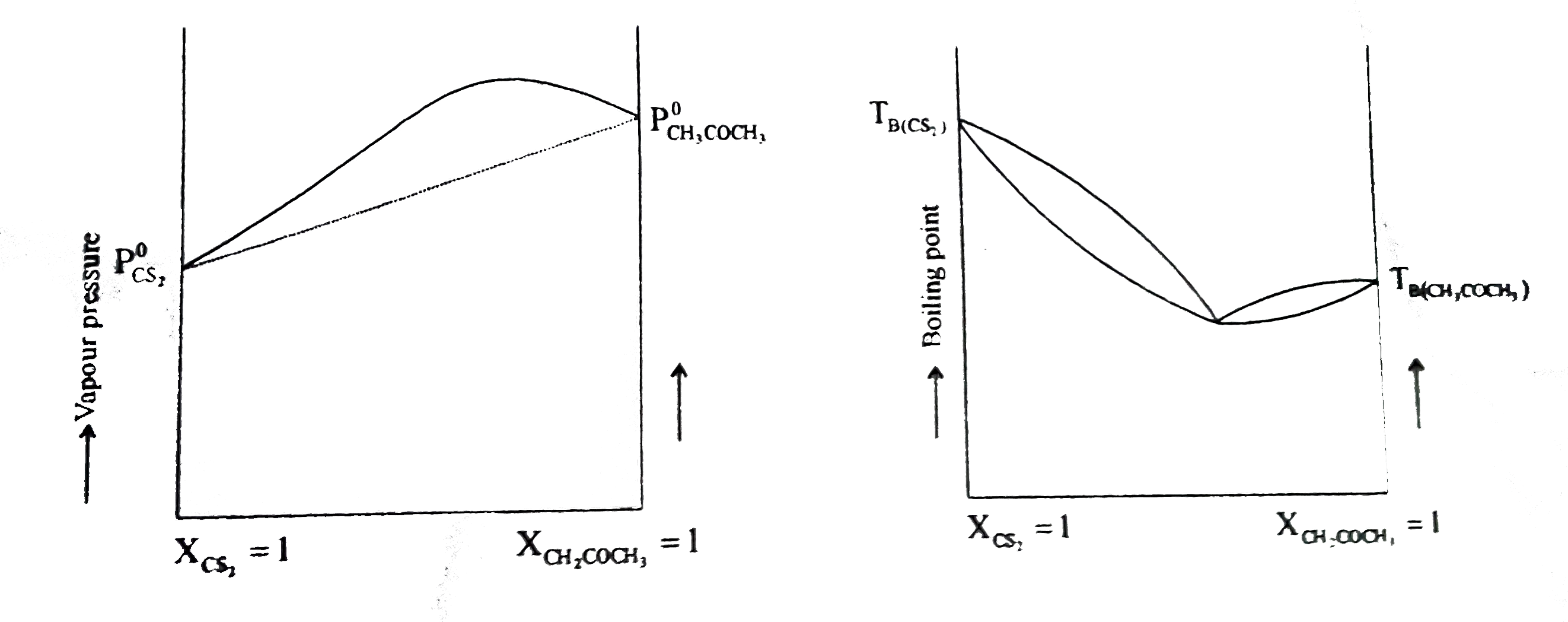
- Acetone and carbon disulphide form binary liquid solution showing pos...
Text Solution
|
- Which of the following solution are isotonic with respect to 0.5 M NaC...
Text Solution
|
- As a gas (insoluable in liquid) is bubbled throuogh a liquid part of t...
Text Solution
|
- Two liquid A and B form an ideal solution. The solution has a vapour p...
Text Solution
|
- A cylinder fitted with a movable piston contains liquid water in equil...
Text Solution
|
- {:(underset(("Colligative properties"))("ColumnI"),underset(("Assume m...
Text Solution
|