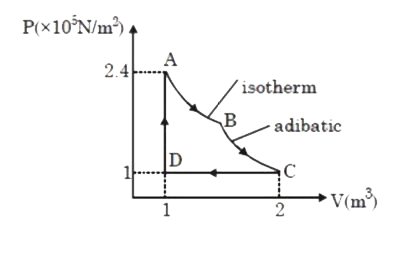
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NEET MAJOR TEST (COACHING)-ALLEN JEE SCORE AIOT TEST 2-PHYSICS
- In the figure shown the key is shorted at time i=0. The initial and th...
Text Solution
|
- Find the root mean square voltage for the variation of voltage as show...
Text Solution
|
- The half-life of a certain radioactive specimen is to be measured. We ...
Text Solution
|
- A block of mass m and relative density y( lt 1) si attached to an idea...
Text Solution
|
- A thin convergent lens is placed between an object and a screen whose ...
Text Solution
|
- When a telescope is adjusted for parallel light, the distance of the o...
Text Solution
|
- Suppose the gravitational force varies inversely as the nth power of d...
Text Solution
|
- 100 mole of an ideal monoatomic gas undergoes a thermodynamic process ...
Text Solution
|
- A shot is fired at an angle theta to the horizontal such that it strik...
Text Solution
|
- A particle moving with velocity v having specific charge (q/m) enters ...
Text Solution
|
- Find the force exerted by a light beam of intensity I, indicated on a ...
Text Solution
|
- A body of mass 1 kg is acclerated uniformly from rest to a speed of 5 ...
Text Solution
|
- When one computes the square root of the ratio of the permeability of ...
Text Solution
|
- Which of the following circuits correctly represents the following tru...
Text Solution
|
- Figure below shows boat and river with their respective speeds. S is a...
Text Solution
|
- A fresnel biprism is used to form the inerference fringes. The distanc...
Text Solution
|
- Determine the change in entropy DeltaS (in SI unit) of 100 gm of water...
Text Solution
|
- In the figure shown, the minimum force F to be applied perpendicular t...
Text Solution
|
- Electric potential in space V(x,y,z) = (x^(2) + y^(2) + z^(2))V Then...
Text Solution
|
- A jet travelling at a constant speed of 1.20 xx 10^(2) m/s executes a...
Text Solution
|