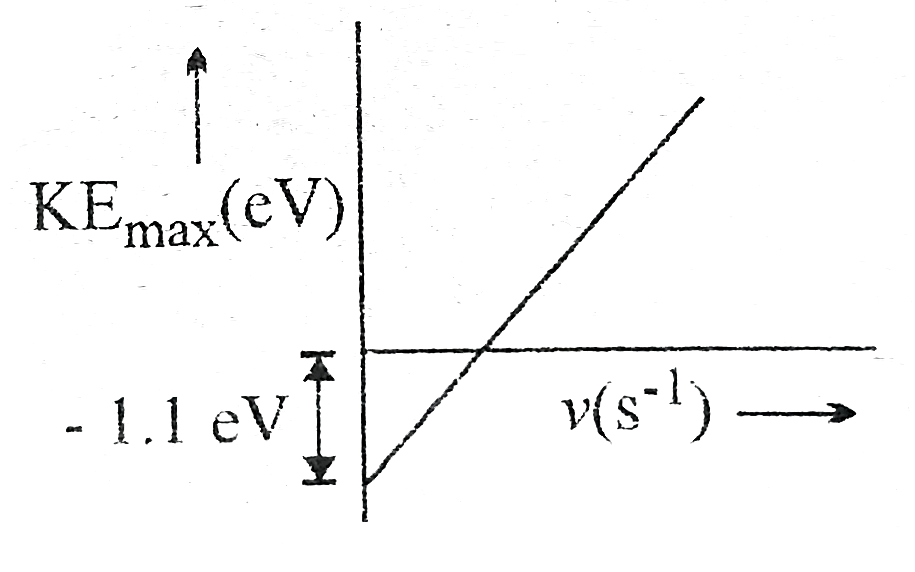Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-ATOMIC STRUCTURE-Solved Examples
- Electromagnetic radiation of wavelength 242 nm is just sufficient t...
Text Solution
|
- What transition in the hydrogen spectrum would have the same wavelen...
Text Solution
|
- Calculate the longest wavelength which can remove the electron from I...
Text Solution
|
- Show that the wavelength of a 150 g rubber ball moving wiith a veloci...
Text Solution
|
- An element undergoes s reaction as show - X+2e^(-)rarrX^(2-), energy ...
Text Solution
|
- light of frequrency 7.5xx10^(14) Hz is incident on a metal surface . A...
Text Solution
|
- How many electron in a given atom can have the following quantum numbe...
Text Solution
|
- What designation will you assign to an to an orbital having following ...
Text Solution
|
- Which of the following set of quantum numbers are not permited (a) n...
Text Solution
|
- (a) for any mutielectron specie, compare the energies of following or...
Text Solution
|
- Calculate energy of electron which is moving in the orbit that has its...
Text Solution
|
- Wavelenght of the Blamer Halpha line is 6565Å. Calculate the wavelengh...
Text Solution
|
- The electron energy in hydrogen atom is given by E(n) =(-21.7xx10^(12)...
Text Solution
|
- Calculate the threshold frequency of metal if the binding energy is 1...
Text Solution
|
- U.V. light of wavelenght 800Å & 700Å falls on hydrongen atoms in thir ...
Text Solution
|
- Though what potential difference must an electron pass to have a wavel...
Text Solution
|
- Calculate magnitude of orbital angular momental of an e^(-) that occup...
Text Solution
|