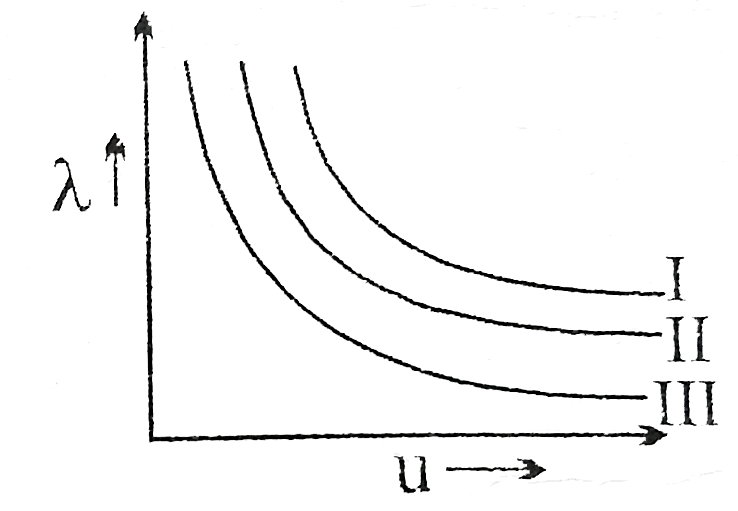
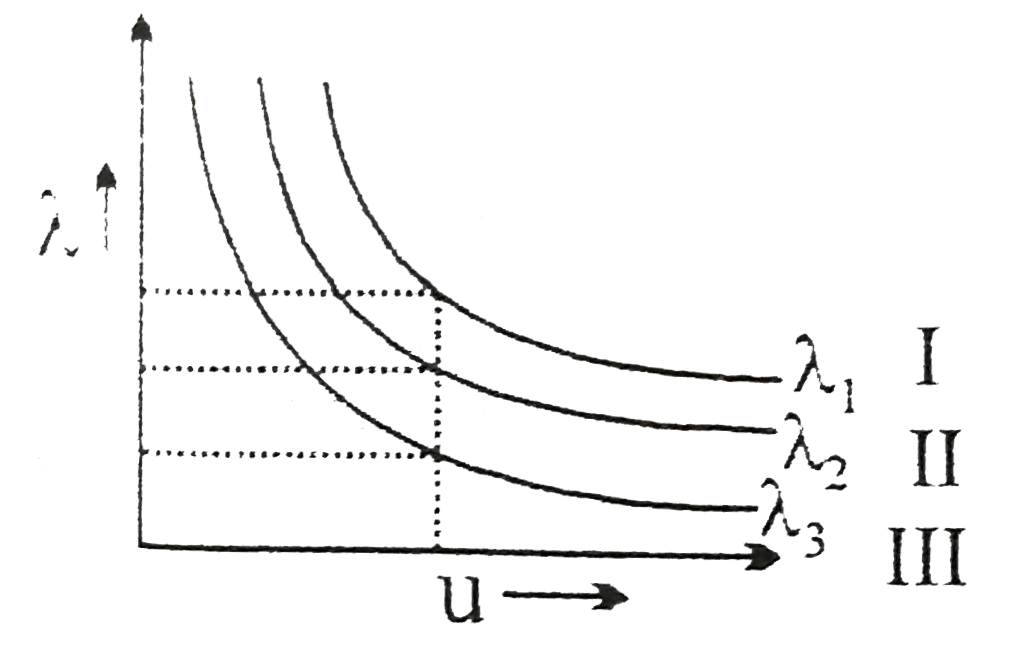
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-ATOMIC STRUCTURE-MULTIPLE CORRECT CHOICE TYPE
- The correct set of possible quantum number number (n,1,m,s,) for las...
Text Solution
|
- Tne number of 'd' electron is Fe^(+2)(Z=26) is equal to that of the
Text Solution
|
- In the following six electronic configuration (remaining inner orbita...
Text Solution
|
- which is are correct statement for following : Mn^(+4),Cr^(+2),Ni^(+...
Text Solution
|
- The radii of two Bhor's orbits of the hydrogen atom are in the ratio ...
Text Solution
|
- Which of the following statement (s) are wrong.
Text Solution
|
- Photons havings 3^(rd) highest energy of this sample can be used to...
Text Solution
|
- The energy of an electron in the first Bohr orbit of H atom is -13.6 e...
Text Solution
|
- Choose the correct statement among the following
Text Solution
|
- Select the correct statement (s):
Text Solution
|
- Choose the incorrect statement (s) :
Text Solution
|
- Select the correct cruve (s) If V=velocity of electron in Bhor's or...
Text Solution
|
- Whichis are correct statement.
Text Solution
|
- Out of the following options in which case in the velocity of electron...
Text Solution
|
- For electron, proton , deuterium molecule(D(2)) & alpha- particule a ...
Text Solution
|
- In a H- like sample electron makes transition from 4th excited state o...
Text Solution
|
- Select correct option(s):
Text Solution
|
- Photons of EMR having wavelenght 469 nm is subjected to a mental sheet...
Text Solution
|
- For He^(+) ion, slect incorrect option (S).
Text Solution
|