Text Solution
Verified by Experts
Topper's Solved these Questions
STATES OF MATTER : GASES AND LIQUIDS
VGS PUBLICATION-BRILLIANT|Exercise LONG ANSWER QUESTIONS|11 VideosSTATES OF MATTER : GASES AND LIQUIDS
VGS PUBLICATION-BRILLIANT|Exercise PROBLEMS|30 VideosSTATES OF MATTER : GASES AND LIQUIDS
VGS PUBLICATION-BRILLIANT|Exercise ADDITIONAL QUESTION & ANSWERS|10 VideosORGANIC CHEMISTRY
VGS PUBLICATION-BRILLIANT|Exercise Long Answer Questions|67 VideosSTOICHIOMETRY
VGS PUBLICATION-BRILLIANT|Exercise ADDITIONAL QUESTIONS & ANSWERS|10 Videos
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-STATES OF MATTER : GASES AND LIQUIDS-SHORT ANSWER QUESTIONS
- State and explain Boyle's law.
Text Solution
|
- State and explain Charle's law.
Text Solution
|
- Derive ideal gas equation.
Text Solution
|
- State and explain Graham's law of Diffusion.
Text Solution
|
- State and explain Dalton's law of partial pressures.
Text Solution
|
- Deduce Boyle's law from kinetic gas equation.
Text Solution
|
- Deduce Charle's kaw from kinetic gas equation.
Text Solution
|
- Deduce Graham's law from kinetic gas equation.
Text Solution
|
- Deduce Dalton's from kinetic gas equation.
Text Solution
|
- Derive an expression for kinetic energy of gas molecules.
Text Solution
|
- Define rms of gas molecule. Give their interrelationship.
Text Solution
|
- Define average of gas molecule. Give their interrelationship.
Text Solution
|
- Define most probable speeds of gas molecule. Give their interrelations...
Text Solution
|
- Explain the physical significance of vander Waals parameter.
Text Solution
|
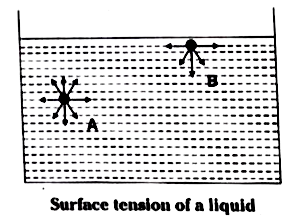
- What is surface tension of liquids? Explain the affect of temperature ...
Text Solution
|
- What is vapour pressure of liquids? How the vapour pressure of a liqui...
Text Solution
|
- Define viscosity and coefficient of viscosity. How does the viscosity ...
Text Solution
|