A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
D-BLOCK ELEMENTS
GRB PUBLICATION|Exercise Comprehension Type|12 VideosD-BLOCK ELEMENTS
GRB PUBLICATION|Exercise Match the column type|6 VideosD-BLOCK ELEMENTS
GRB PUBLICATION|Exercise Subjective Type|18 VideosCOORDINATION COMPOUNDS
GRB PUBLICATION|Exercise SBJECTIVE TYPE|98 VideosELECTROCHEMISTY
GRB PUBLICATION|Exercise Subjective type|39 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-D-BLOCK ELEMENTS-Multiple objective type
- Select the correct statement(s) for interstitial compounds.
Text Solution
|
- Select the correct reaction for vanadium species:
Text Solution
|
- Select the correct statement(s) about Cr(2)O(7)^(2-) and CrO(4)^(2-)
Text Solution
|
- Select the correct reaction sequence(s)?
Text Solution
|
- Choose the correct statement about KMnO(4).
Text Solution
|
- Conversion of manganous salt to MnO(2) using KMnO(4) in netural medium...
Text Solution
|
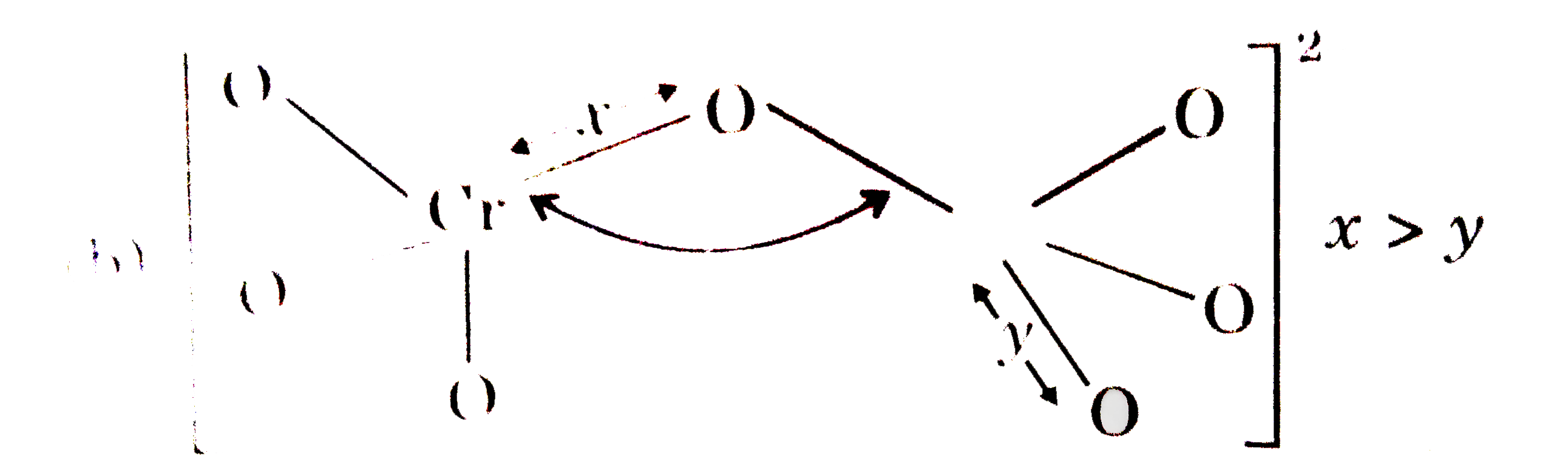
- An element of 3d-transition series shows two oxidation states x and y,...
Text Solution
|
- To an acidified dichromate solution, a pinch of Na(2)O(2) is added and...
Text Solution
|
- Potash alum is a double salt, its aqueous solution shows the character...
Text Solution
|
- Addition of non-metals like B and C to the interstitial sites of a tra...
Text Solution
|
- Mercury is a liquid at 0^(@) C because of
Text Solution
|
- The correct statement(s) about transition element is/are.
Text Solution
|
- The ionisation energies of tranition elements are:
Text Solution
|
- The metal(s) which does/do not form amalgam is/are:
Text Solution
|
- Which of the following statements concern with d-block metals?
Text Solution
|
- The highest oxidation state exhibited by a transition elements is .
Text Solution
|
- Amphoteric oxide(s) is/are
Text Solution
|
- The catalytic activity of transition elements is related to their:
Text Solution
|
- In the equation: M+8CN^(-)+2H(2)O+O(2)to4[M(CN)(2)]^(-)+4OH^(-) meta...
Text Solution
|
- CuSO(4)(aq)+4NH(3)toX, then X is
Text Solution
|