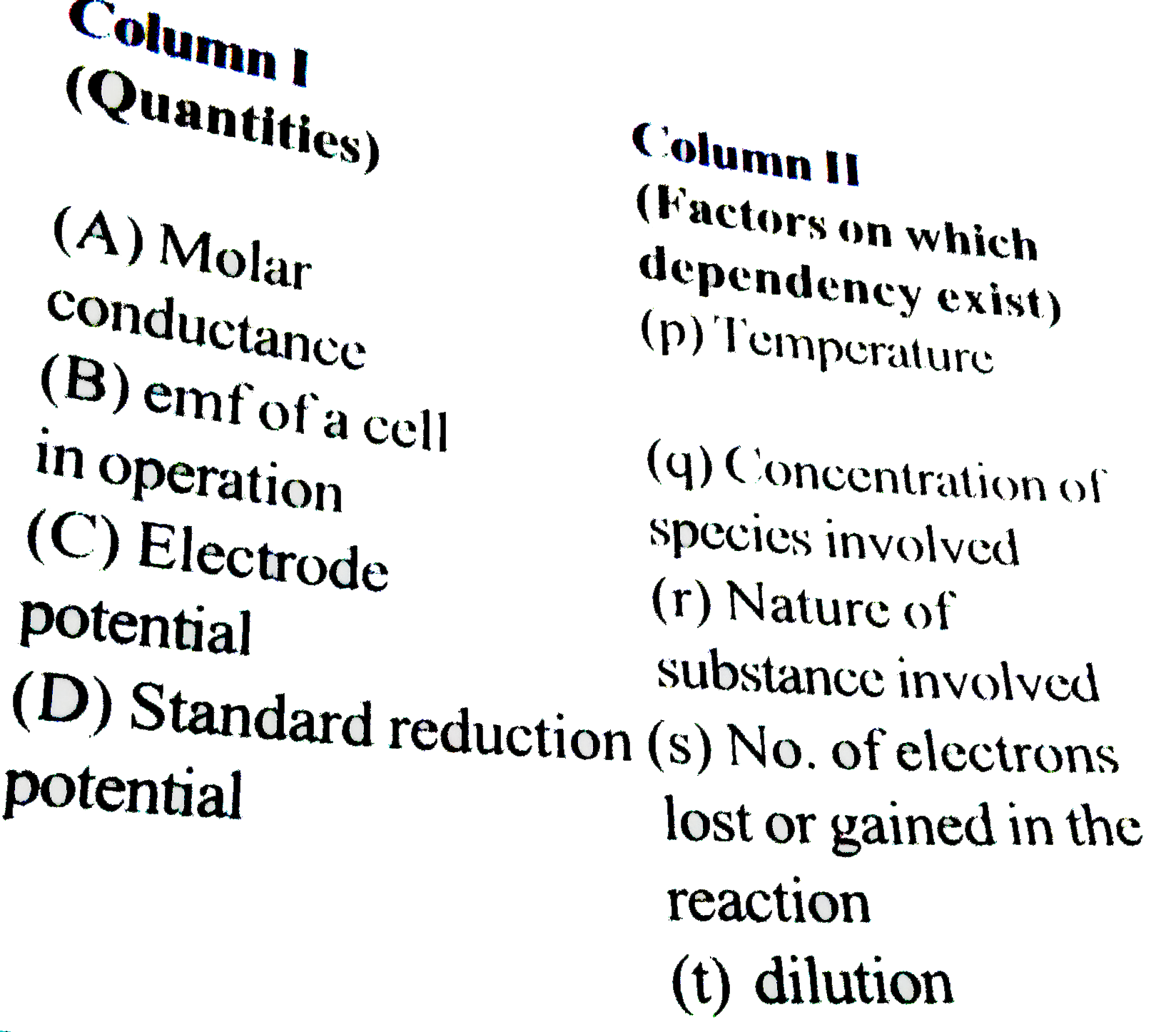Text Solution
Verified by Experts
The correct Answer is:
NARAYNA- ELECTRO CHEMISTRY-LEVEL-V
- The concentration of potassium ions inside a biological cell is at lea...
Text Solution
|
- The electrochemical cell shown below is a concentration cell M//M^(...
Text Solution
|
- The electrochemical cell shown below is a concentration cell. M|M^(2+)...
Text Solution
|
- For the galvanic cell Ag|Ag((aq))^(+)(0.1M)"||"Cd((aq))^(2+)(0.1M)|C...
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- Statement : The cathode of electrolytic cell during electrolysis of Na...
Text Solution
|
- Statement : Pt(H(2))|HCl at 25^(@)C E(H) = 0. Explanation : For pri...
Text Solution
|
- Statement : ((deltaE)/(deltaT))(P) is called temperature coefficient o...
Text Solution
|
- Statement : Liquid juncation potential can be eliminated by putting a ...
Text Solution
|
- Assertion(A): Whne acidified ZnSO(4) solution is electrolyzed between ...
Text Solution
|
- Assertion (A): The mobility of Na^(o+) is lower than that of K^(o+) io...
Text Solution
|
- Statement : E(cell)^(@) is an intensive property Explanation : Delta...
Text Solution
|
- Statement : If two half reaction with electrode potential E(1)^(@) and...
Text Solution
|
- Find the volume of Cl(2) at NTP produced during electrolysis of MgCl(2...
Text Solution
|
- The standard oxidation potential of Ni//Ni^(2+) electrode is 0.236 V. ...
Text Solution
|
- A hydrogen electrode is dipped in a solution at 25^(@)C. The potential...
Text Solution
|
- What current is to be passed for 0.25 sec for deposition of certain we...
Text Solution
|
- When water is electrolysed, hydrogen and oxygen gas are produced. If 1...
Text Solution
|