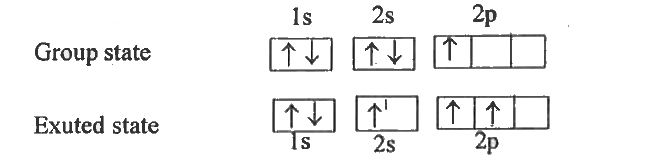
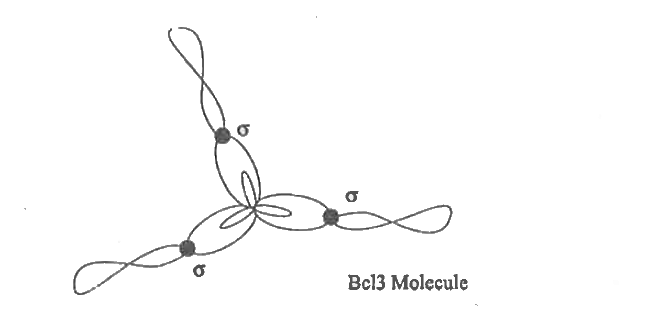
Text Solution
Verified by Experts
Topper's Solved these Questions
ANNUAL EXAMINATION QUESTION PAPER SOUTH-2017
SUBHASH PUBLICATION|Exercise PART-D|19 VideosANNUAL EXAMINATION QUESTION PAPER SOUTH-2017
SUBHASH PUBLICATION|Exercise PART-E|6 VideosANNUAL EXAMINATION QUESTION PAPER SOUTH-2017
SUBHASH PUBLICATION|Exercise PART-B|9 VideosANNUAL EXAMINATION QUESTION PAPER NORTH-2019
SUBHASH PUBLICATION|Exercise PART-D|27 VideosANNUAL EXAMINATION QUESTION PAPER SOUTH-2018
SUBHASH PUBLICATION|Exercise PART-E|7 Videos
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-ANNUAL EXAMINATION QUESTION PAPER SOUTH-2017-PART-C
- Write a brief note on s,p and d block elements.
Text Solution
|
- Explain Sp^(2) hybridisation taking boron trichloride (BCl(3)) as an e...
Text Solution
|
- Write any three postulates of VSEPR theory.
Text Solution
|
- Write the molecular orbital electronic configuration for carbon monoxi...
Text Solution
|
- Balance the redox reaction by oxidation number method. MnO(4)^(-)(aq...
Text Solution
|
- Complete the reaction: C(s)+H(2)O(g)overset(Delta)(to)
Text Solution
|
- Complete the reaction: CO(g)+H(2)O(g)overset(Delta)(to)
Text Solution
|
- Complete the reaction: Zn(s)+2H^(+)(aq)to
Text Solution
|
- Write the equations during the preparation of sodium carbonate by solv...
Text Solution
|
- Graphite is a good conductor of electricity. Give reason.
Text Solution
|
- Give the chemical formula of borazine.
Text Solution
|
- Complete the equation HCOOHunderset(373k)overset(conc.H(2)SO(4))(to)
Text Solution
|