A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise Level 2 (Q.26 To Q.50)|25 VideosGENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise Level 2 (Q.51 To Q.75)|25 VideosGENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise Level 1 (Q.26 To Q.49)|24 VideosCHEMISTRY IN DAILY LIFE
HIMANSHU PANDEY|Exercise Integer Answer Type Problems|7 VideosHALIDES
HIMANSHU PANDEY|Exercise Subjective Type Problems|10 Videos
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-GENERAL ORGANIC CHEMISTRY-Level 2 (Q.1 To Q.25)
- In which of the following pairs is the sturcture on the right major re...
Text Solution
|
- In which of the following pairs of resonance contributors is the sturc...
Text Solution
|
- Examine the following resonating structures of formic acid and arrange...
Text Solution
|
- Which of the following compounds will not show resonance?
Text Solution
|
- Which of the following compounds will exhibit d-orbital resonance?
Text Solution
|
- Which of the following represents resonating structure of
Text Solution
|
- Which of the following is not a permissible resonating form?
Text Solution
|
- Less contributing structure of nitroethene is :
Text Solution
|
- Which will be the least stable resonating structure?
Text Solution
|
- Which will be the least stable resonating structure?
Text Solution
|
- Which of the following pairs of structures is not a pair of resonating...
Text Solution
|
- Which of the following pairs of strutures does not represent resonatin...
Text Solution
|
- The most stable resonating structure of p-nitrosonitrobenzene is :
Text Solution
|
- Which of the following molecules has longest C=C strenght?
Text Solution
|
- The decreasing order of bond length of C=C in the following compounds ...
Text Solution
|
- In which of the following molecules all the effects namely inductive, ...
Text Solution
|
- There are three canonical structures of naphthalene. Examine them and ...
Text Solution
|
- Among these compounds the correct order of C-N bond length is :
Text Solution
|
- C(1)-C(2) bond is shortest in :
Text Solution
|
- Among the following three canonical sturetures what would be their rel...
Text Solution
|
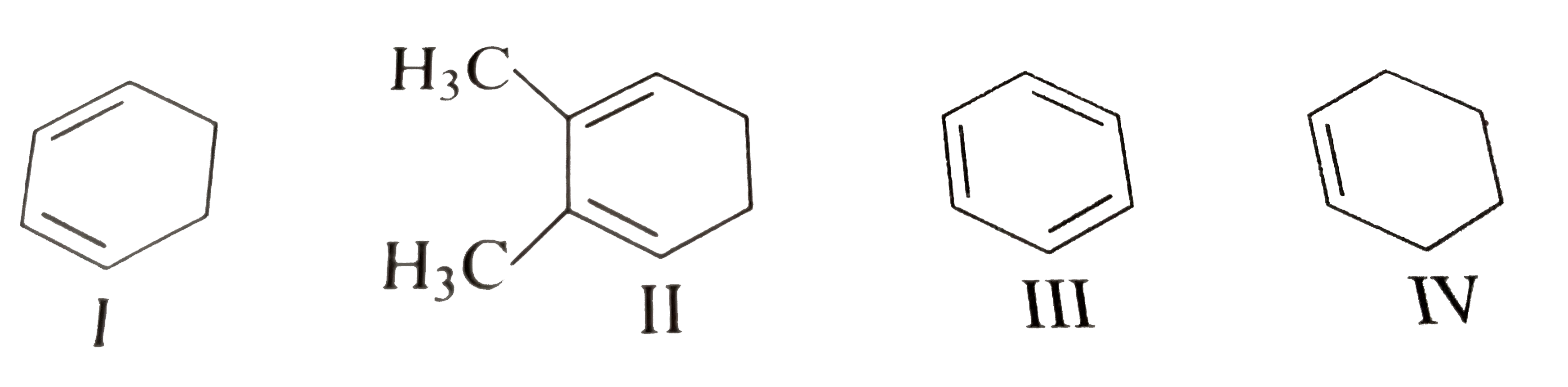
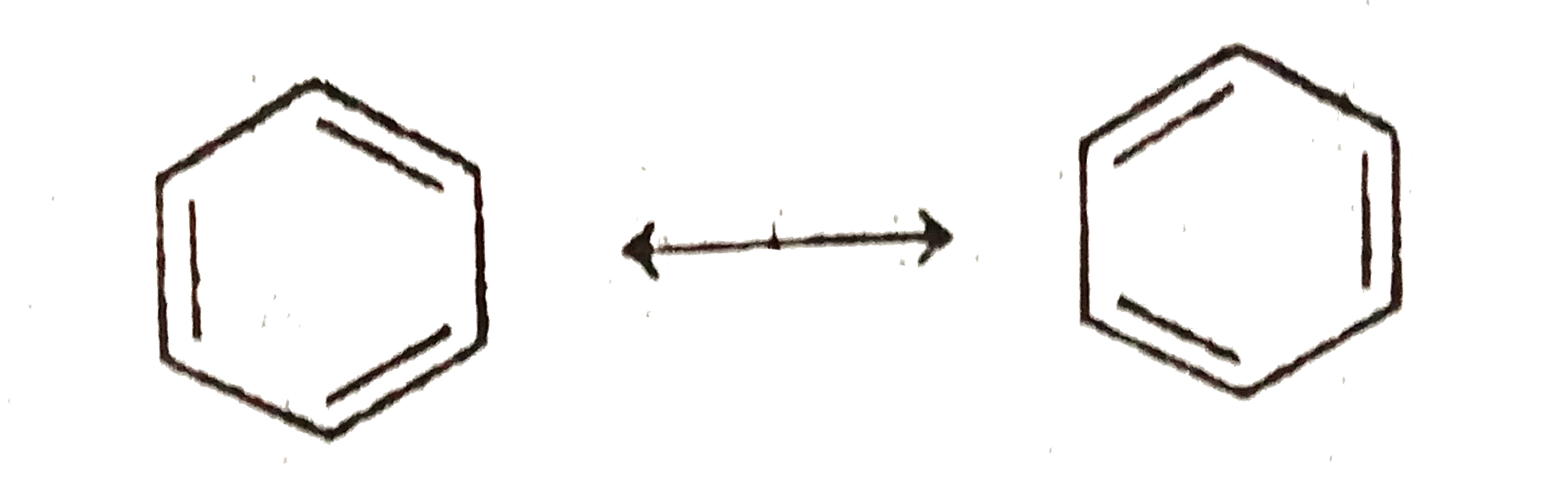 resonance also occurs in (I)
resonance also occurs in (I)