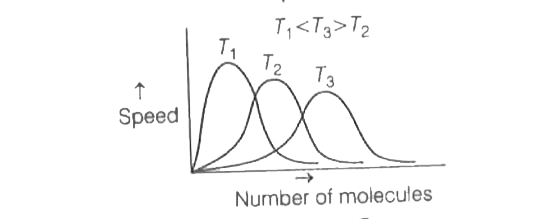A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
TS EAMCET PREVIOUS YEAR PAPERS-TS EAMCET 2018 (7 MAY SHIFT 1)-CHEMISTRY
- The bond orders of He (2) ^(+)and He (2) are respectively
Text Solution
|
- Kinetic energy in Kj of 280 g of N(2) at 27^(@)C is approximately (R ...
Text Solution
|
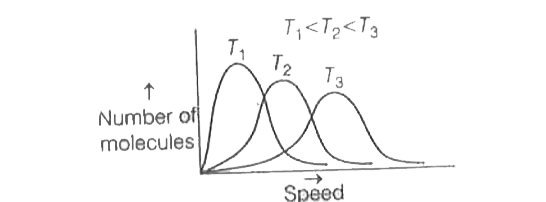
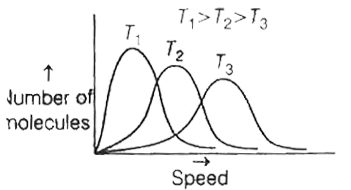
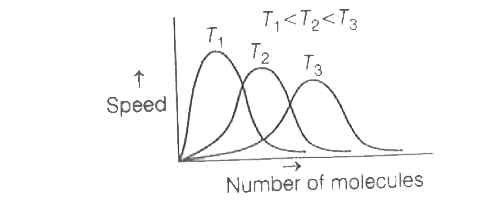
- The correct plot of Maxweel-Boltzmann distribution at different temper...
Text Solution
|
- CaCO(3) reacts with HCl to produce CaCl (2), CO(2)and H (2)O. The appr...
Text Solution
|
- Calculate the approximate mas (in g ) of H (2)S required for the follo...
Text Solution
|
- For the following process H (2)O (l) (1 bar 373.15K) hArr H(2)O (g) ...
Text Solution
|
- PCl (5) hArr PCl (3) + PCl(3) If the equilibrium cnstant (K (c)) for...
Text Solution
|
- What is the pH of acetic acid at equilibrium, given that acetic acid c...
Text Solution
|
- Assertion (A) Ferricyanide ion oxidises H (2) O (2) to H (2)O in basic...
Text Solution
|
- Highest melting point among the following is displayed by
Text Solution
|
- Diborane reacts with ammonia to from X, which on heating gives H (2) a...
Text Solution
|
- The stability of dihalides of Si,Ge, Sn and Pb follows the sequence.
Text Solution
|
- Which of the following statements about smog is/are correct ? (i) S...
Text Solution
|
- Which of the following statements about TLC are correct ? (i) Glycin...
Text Solution
|
- Identify X,Y and Z in the following reactions
Text Solution
|
- Which of the following statements are correct with respect to benzene ...
Text Solution
|
- A compound having elements X and Y crystallises in a cubic structure, ...
Text Solution
|
- If the degree of association is 70% for the reaction 2A hArr (A) (2) t...
Text Solution
|
- 0.1 mole of NaCl is dissolved in 100g of water. The mole fraction of N...
Text Solution
|
- What is the approximate standard free energy change per mole of Zn (in...
Text Solution
|