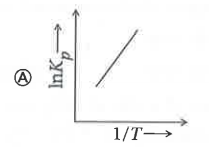
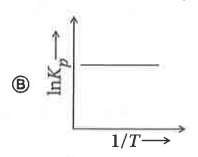
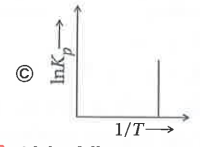
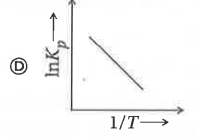
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
EQUILIBRIUM
CHHAYA PUBLICATION|Exercise SINGLE CORRECT TYPE (MCQ HOTSPOT)|84 VideosEQUILIBRIUM
CHHAYA PUBLICATION|Exercise VERY SHORT TYPE QUESTIONS|26 VideosEQUILIBRIUM
CHHAYA PUBLICATION|Exercise HIGHER ORDER THINKING SKILL (HOTS) QUESTIONS|17 VideosENVIRONMENTAL CHEMISTRY
CHHAYA PUBLICATION|Exercise PRACTICE SET 14(Answer the following questions)|6 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
CHHAYA PUBLICATION|Exercise PRACTICE SET 6|10 Videos
Similar Questions
Explore conceptually related problems
CHHAYA PUBLICATION-EQUILIBRIUM-ENTRANCE QUESTIONS BANK
- In which of the following mixed aqueous solutions pH=kea at equilibriu...
Text Solution
|
- The molar solubility (in mol L^(-1)) of a sparingly soluble salt MX4 i...
Text Solution
|
- Which of the following plots represents an exothermic reaction-
Text Solution
|
- Of the following compounds, which one is the strongest acid in an aqu...
Text Solution
|
- Dissolving NaCN in demonized water will result in a solution having
Text Solution
|
- Equilibrium constants for the following reactions at 1200 K are given ...
Text Solution
|
- Your are supplied with 500 mL each of 2(N) HCl and 5(N) HCl. What is t...
Text Solution
|
- The following euilibrium constants are given: N2+3H2<implies2NH3,K1"...
Text Solution
|
- A vessel at 1000K contains CO2 with a pressure of 0.5 atm. Some of the...
Text Solution
|
- The equilibrium constant (Kc) for the reaction N2(g)+O2(g)to2NO(g) at ...
Text Solution
|
- The pH of a 0.1 molar solution of the acid HQ is 3. The value of the i...
Text Solution
|
- How many litres of water mutt be added to 1 litre of of an aqueous sol...
Text Solution
|
- The molarity of a solution obtained by mixing 750 mL of 0.5(M) HCI wit...
Text Solution
|
- For the reaction SO2(g)+1/2O2(g)<impliesSO3(g), if kp=cc(RT)^x where t...
Text Solution
|
- The following reaction is performed at 298K 2NO(g)+O2(g)hArr2NO2(g) ...
Text Solution
|
- The standard Gibbs energy change at 300K for the reaction 2A⇋B+C is 24...
Text Solution
|
- The equilibrium constant at 298 K for a reaction, A+B⇌C+D is 100. If t...
Text Solution
|
- pKa of a weak acid (HA) and pKb of a weak base (BOH) are 3.2 and 3.4, ...
Text Solution
|
- Which of the following salts is most basic in aqueous solution-
Text Solution
|
- An alkali is titrated against an acid with methyl orange as indicator....
Text Solution
|