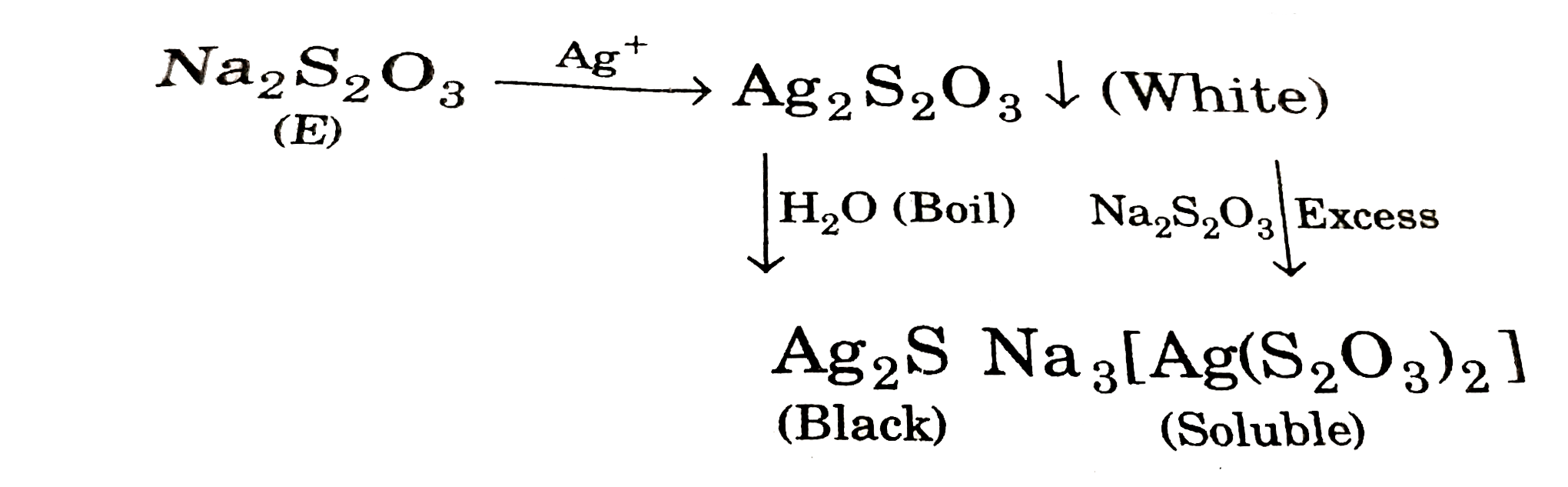A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SALT ANALYSIS
GRB PUBLICATION|Exercise F.Zero Group Cations|18 VideosSALT ANALYSIS
GRB PUBLICATION|Exercise G: Ist Group Cations|20 VideosSALT ANALYSIS
GRB PUBLICATION|Exercise D.Class-A Subgroup-II Acidic Radicals|39 VideosS BLOCK ELEMENTS
GRB PUBLICATION|Exercise SUBJECTIVE TYPE|9 VideosTHERMODYNAMICS
GRB PUBLICATION|Exercise All Questions|704 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-SALT ANALYSIS-E.Precipitation/Redox Reacttions
- Identify the correct statement:
Text Solution
|
- Anion which is protonated by dil H(2)SO(4) but produce volatile produc...
Text Solution
|
- Silver nitrate solution when added to a colourless aqueous solution E ...
Text Solution
|
- When an inorganic compound reacts with SO2 in aqueous medium produces ...
Text Solution
|
- On the addition of a solution containing CrO(4)^(2-) ions to the solut...
Text Solution
|
- Identify the salt (X) which when treated with K(2)CrO(4) in the presen...
Text Solution
|
- AgCl is soluble in NH(4)OH solution. The solubility is due to the form...
Text Solution
|
- An unknown anion in solution is to be identified by adding Ag^(+) and ...
Text Solution
|
- What happens when 6M nitric acid is added to an aqueous solution that ...
Text Solution
|
- A mixture of which 0.2M aqueous solutions will form a precipitate the ...
Text Solution
|
- Which pair of aqueous solutions produce a yellow precipitate upon mixi...
Text Solution
|
- An aqueous solution is known to contain Ag^(+),Mg^(2+) and Sr^(2+) ion...
Text Solution
|
- Mixing which pair of 0.10M solutions produces two precipitates that ca...
Text Solution
|
- An aqueous solution contains the ions Ag^(+),Ba^(2+), and Ni^(2+). Dil...
Text Solution
|
- When equal volumes of 0.2M solutions of the following compounds are mi...
Text Solution
|
- Mixing equal volumes of 0.1MCa(NO(3))(2) and AgF solution results in:
Text Solution
|
- In above reaction amyl alcohol is recommended. Dimethyl ether is not r...
Text Solution
|
- The yellow colour solution of Na(2)CrO(4) changes to orange red on pas...
Text Solution
|
- White precipitate of AgCl turns to greyish or black when:
Text Solution
|
- Tha gas G will show which of the following property?
Text Solution
|