Text Solution
Verified by Experts
Topper's Solved these Questions
ALDEHYDES AND KETONES
DINESH PUBLICATION|Exercise Question From Board|86 VideosALDEHYDES AND KETONES
DINESH PUBLICATION|Exercise Higher Order Thinking Skills|6 VideosALDEHYDES AND KETONES
DINESH PUBLICATION|Exercise Problem|15 VideosALCOHOLS AND PHENOLS
DINESH PUBLICATION|Exercise Matrix|9 VideosALKALI EARTH METALS
DINESH PUBLICATION|Exercise Unit test-12|5 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-ALDEHYDES AND KETONES -Additional Important Question
- Out of benzaldehyde and propionaldehyde, which is mre reactive towards...
Text Solution
|
- Boiling points of ketones are higher than those of the isomeric aldehy...
Text Solution
|
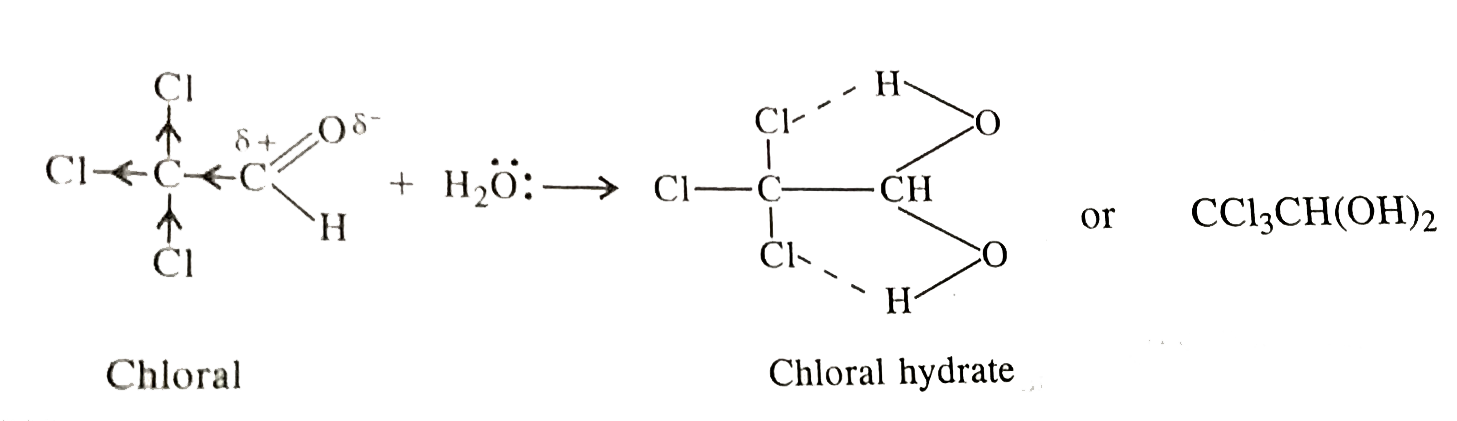
- Chloral hydrate is a gem-diol but still stable. How will you account f...
Text Solution
|
- Ketones are less reactive than aldehydes in the nucleophilic addition ...
Text Solution
|
- Why does not formaldehyde take part in aldol condensation?
Text Solution
|
- Why is it necessary to control the pH during the reaction of aldehydes...
Text Solution
|
- Benzophenone does not react with NaHSO(3). Explain.
Text Solution
|
- Why does formaldehyde undergo Cannizzaro's reaction while acetaldehyde...
Text Solution
|
- How wil you convert an alkene into an aldehyde containing one carboon ...
Text Solution
|
- Outline the synthesis of PhCH(2)CH(2)CHO from benzene, ethylene oxide ...
Text Solution
|
- How will you bring about the following changes :
Text Solution
|
- Arrange the following in the order of their incresing reactivity towar...
Text Solution
|
- Arrange the following compounds in order of increasing reactivity towa...
Text Solution
|
- Name two reagents which can be used to convert gtC = O to gt CH(2) gro...
Text Solution
|
- Which alkene upon reductive ozonolysis will give acetone as the produc...
Text Solution
|
- Upon ozonolysis, molecule of a hydrcarbon produces of ethanal and one ...
Text Solution
|
- Why a benzaldehyde less reactive than acetaldehyde towards nucleophili...
Text Solution
|
- Formaldehyde gives Cannizzaro's reaction while acetaldehyde does not....
Text Solution
|
- What happens when ethanal is treated with methyl magnesium iodide foll...
Text Solution
|
- Identify the compounds [E] to [J] in the following reactions. {:([E]...
Text Solution
|