Text Solution
Verified by Experts
Topper's Solved these Questions
ALDEHYDES AND KETONES
DINESH PUBLICATION|Exercise Higher Order Thinking Skills|6 VideosALDEHYDES AND KETONES
DINESH PUBLICATION|Exercise Practice Test|7 VideosALDEHYDES AND KETONES
DINESH PUBLICATION|Exercise Additional Important Question|35 VideosALCOHOLS AND PHENOLS
DINESH PUBLICATION|Exercise Matrix|9 VideosALKALI EARTH METALS
DINESH PUBLICATION|Exercise Unit test-12|5 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-ALDEHYDES AND KETONES -Question From Board
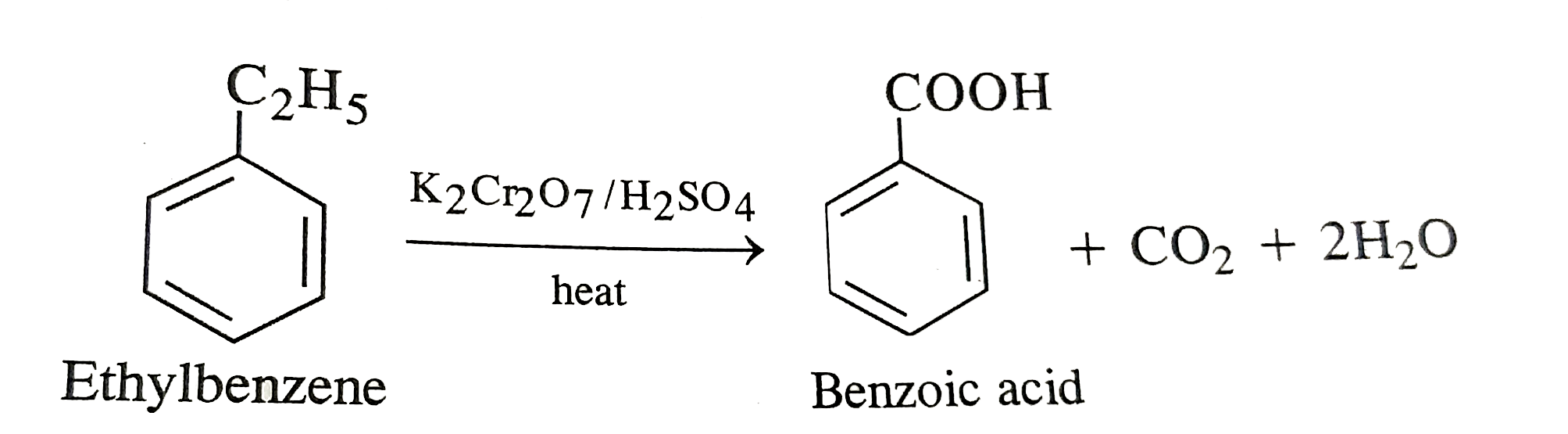
- What happens when ethylbenzene is heated with acidified K(2)Cr(2)O(7) ...
Text Solution
|
- Why are aldehydes more reavtice than ketones towards nucleophilic addi...
Text Solution
|
- Convert acetone to teriary butyl alcohol?
Text Solution
|
- What is Tollen's reagent?
Text Solution
|
- How are formalin and trioxane related to methanal?
Text Solution
|
- Write chemical equation to illustrate Rosenmund's reduction.
Text Solution
|
- How will you perpare ethlamine from acetaldehyde ?
Text Solution
|
- How will you convert ethanol to propanone ?
Text Solution
|
- Give a chemical test to distinguish between benzaldehyde and acetone.
Text Solution
|
- Give a chemical test to distinguish between methanol and ethanol.
Text Solution
|
- Write IUPAC name of the compound CH(3)-underset(CH(3))underset(|)(CH)-...
Text Solution
|
- Write a test to differentiate between pentan-2-one and pentan-3-one.
Text Solution
|
- How will you convert acetaldehyde into methane?
Text Solution
|
- Write IUPAC name of the compound : CH(3)COCH(2)COCH(3).
Text Solution
|
- Write the chemical equation for each of the following reactions : (i...
Text Solution
|
- How will you convert ethanol to propanone ?
Text Solution
|
- Complete the followning : (i) CH(3)COCH(3) + HI overset("Red"P//423K...
Text Solution
|
- Complete the following with appropriate reagents
Text Solution
|
- How many asymmetric carbon atoms are created during the complete reduc...
Text Solution
|
- Complete the following (i) 3(CH(3))(2)C = Ooverset([A])rarr (ii) C...
Text Solution
|