Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-SOME BASIC CONCEPTS OF CHEMISTRY-All Questions
- Distinguish between molarity and molality.
Text Solution
|
- Calculate the mass per cent of calcium, phosphorus and oxygen in calci...
Text Solution
|
- 45.4L of dinitrogen reacted with 22.7L of dioxygen and 45.4 L of nitro...
Text Solution
|
- If two elements can combine to form more than one compound, the masses...
Text Solution
|
- Calculate the average atomic mass of hydrogen using the following data...
Text Solution
|
- Hydrogen gas is prepared in the laboratory by reacting dilute HCl with...
Text Solution
|
- The density of 3 molal solution of NaOH is 1.110g mL^(-1). Calculate t...
Text Solution
|
- Volume of a solution chagnes with chagne in temperature, then what wil...
Text Solution
|
- If 4 g of NaOH dissovles in 36g of H(2)O, calculate the mole fraction ...
Text Solution
|
- The reactant which is entirely consumed in reaction is known as limiti...
Text Solution
|
- Match the following.
Text Solution
|
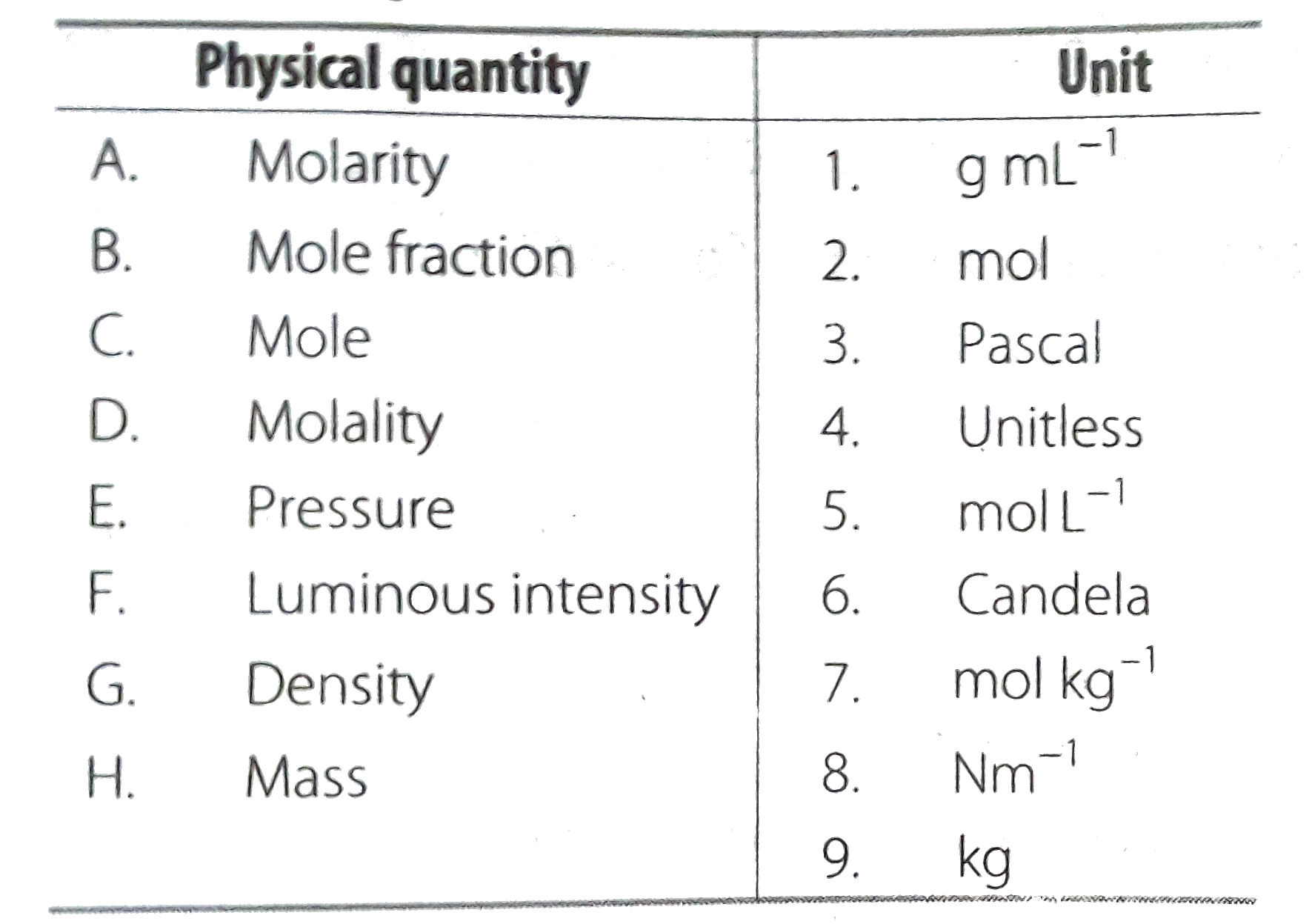
- Match the following physical quantities with units.
Text Solution
|
- Assertion(A) The empirical mass of ethene is half of its molecular mas...
Text Solution
|
- Assertion(A) One atomic mass unit is defined as one twelth of the mass...
Text Solution
|
- Assertion(A) Significant figures for 0.200 is 3 where as for 200 it is...
Text Solution
|
- Assertion(A) Combustion of 16g of methane give18 g of water. Reason(...
Text Solution
|
- A vessel contains 1.6g of dioxygen at STP(273.15k,1atm pressure). The ...
Text Solution
|
- Calcium carbonate reacts with aqueous HCl to give CaCl(2) and CO(2) a...
Text Solution
|
- Define the law of multiple proportions, Explain it with two examples. ...
Text Solution
|
- A b ox contains some identical red coloured balls. Labelleda as A, eac...
Text Solution
|