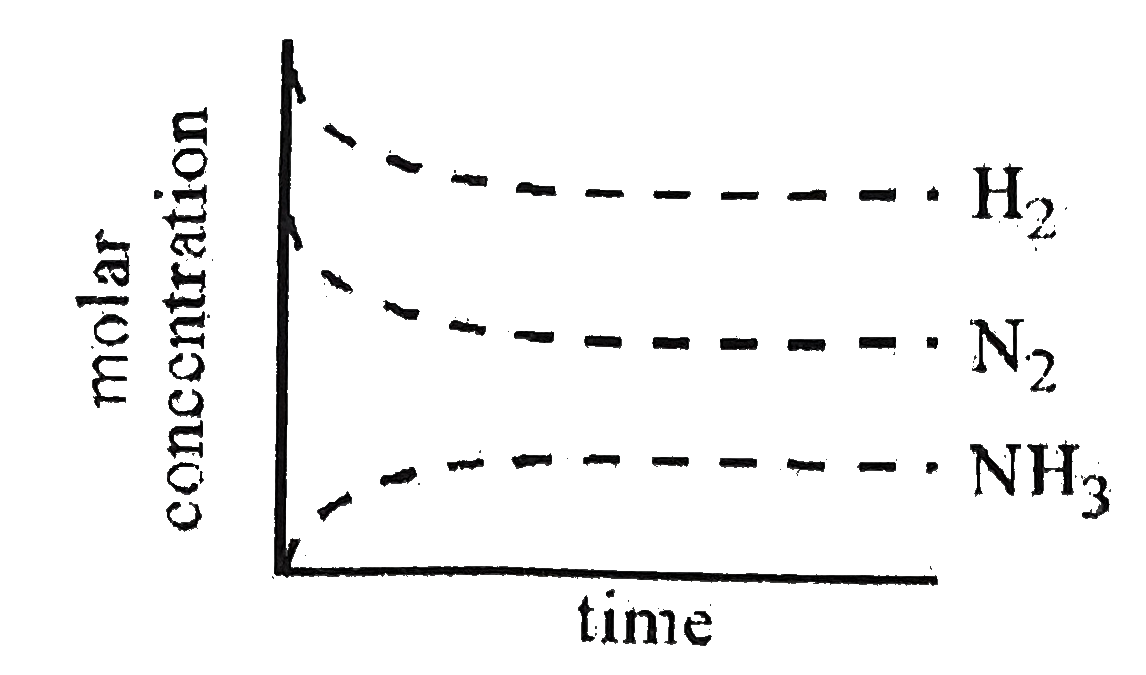
A
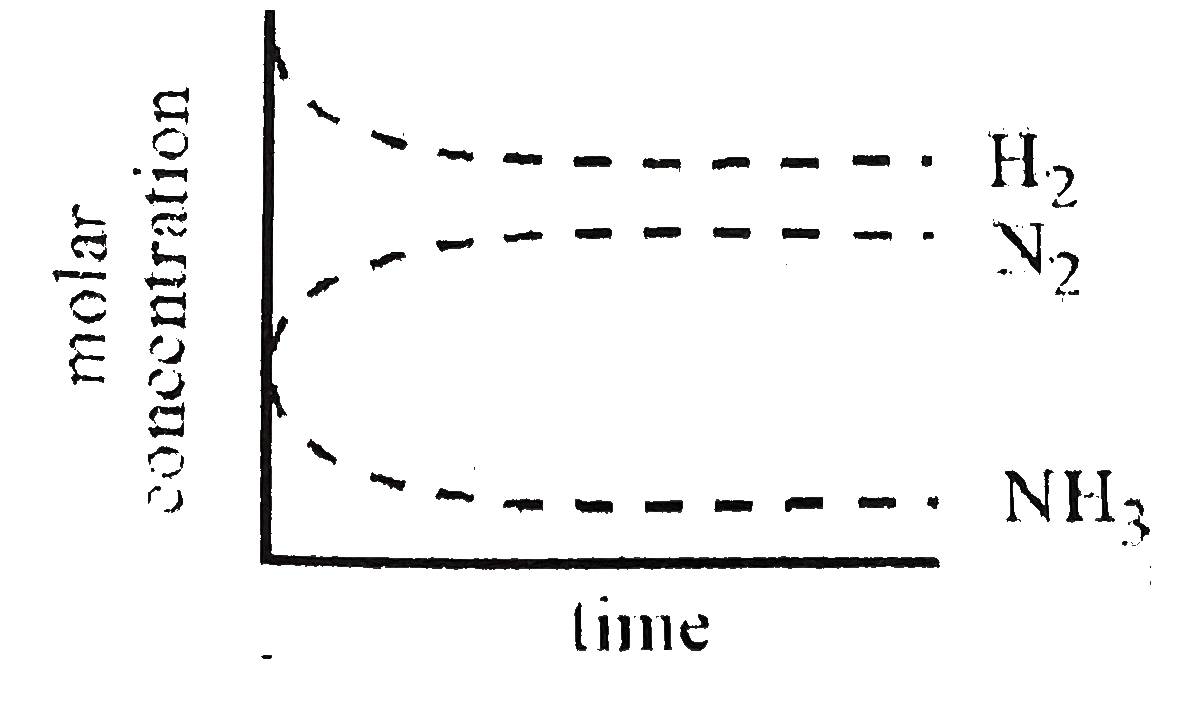
B
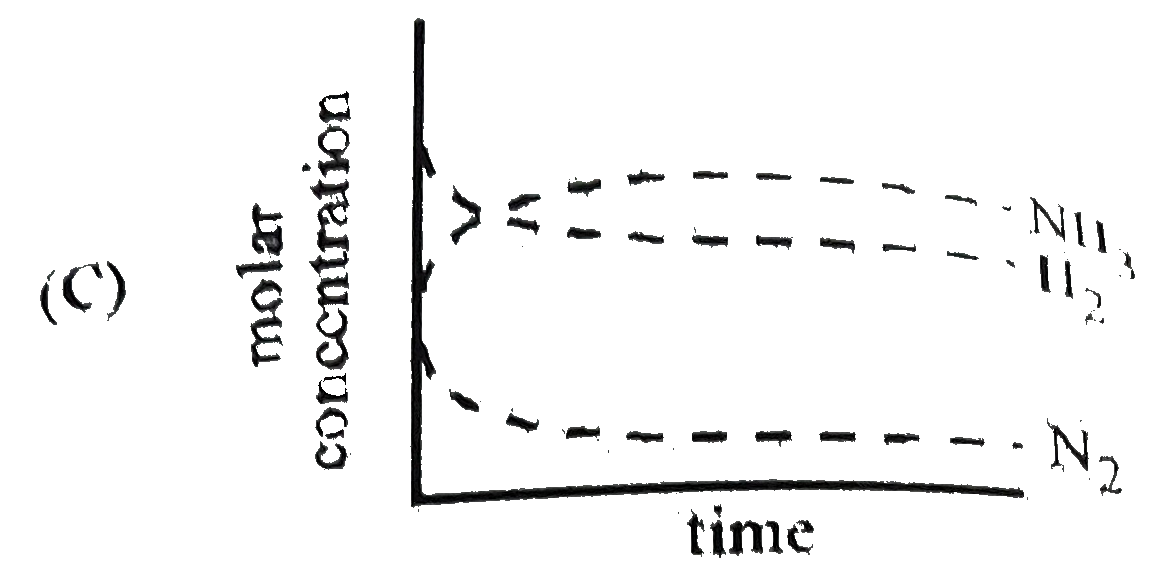
C
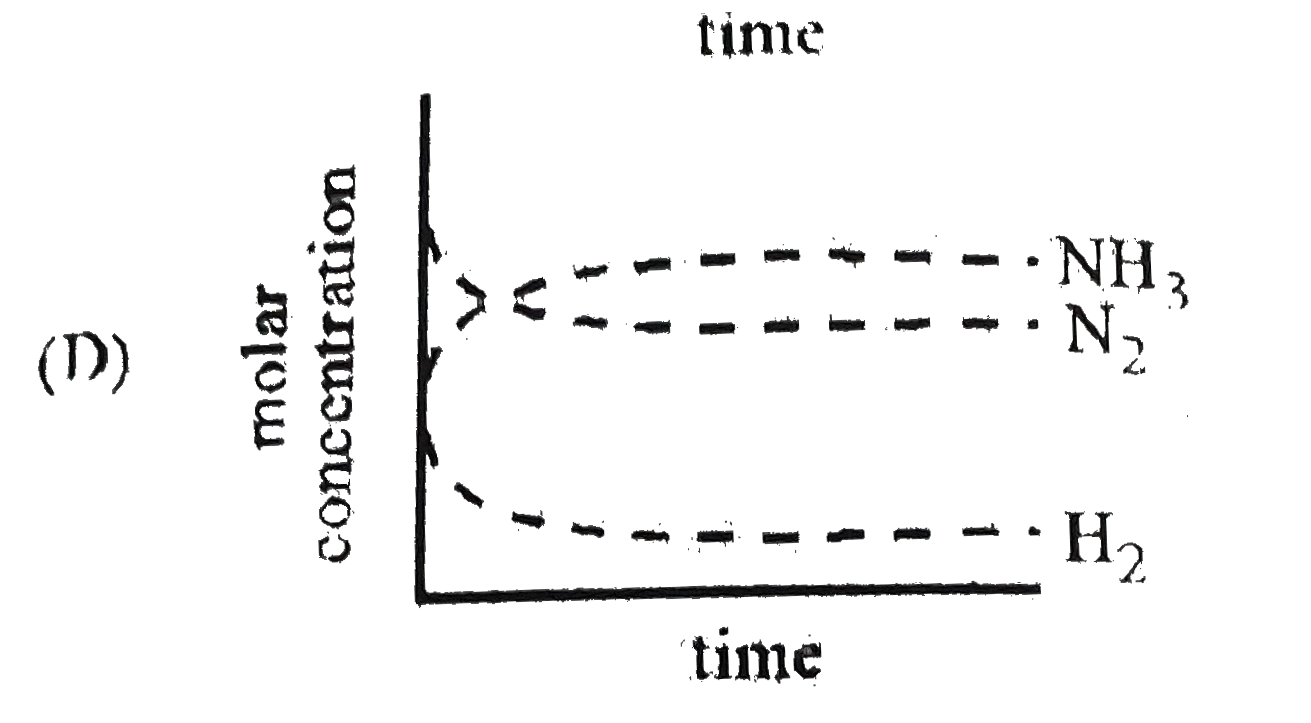
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PHYSICAL AND CHEMICAL EQUILIBRIA
DINESH PUBLICATION|Exercise SELECTED STRAIGHT OBJECTIVE TYPE MCQs|4 VideosPHYSICAL AND CHEMICAL EQUILIBRIA
DINESH PUBLICATION|Exercise MCQs with only one correct answer|24 VideosPHYSICAL AND CHEMICAL EQUILIBRIA
DINESH PUBLICATION|Exercise Multiple CHOICE QUESTIONS [Based on Numerical Problems]|62 VideosP BLOCK ELEMENTS (GROUP 13 AND 14 )
DINESH PUBLICATION|Exercise Straight obj.|17 VideosRATES OF REACTIONS AND CHEMICAL KINETICS
DINESH PUBLICATION|Exercise Ultimate Preparatory Package|29 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-PHYSICAL AND CHEMICAL EQUILIBRIA-REVISION QUESTION FROM COMPETITIVE EXAMS
- In the reaction PCl(5)(g) hArr PCl(3)(g)+Cl(2)(g), the equilibrium c...
Text Solution
|
- One mole of a compound AB reacts with 1 mole of a compound CD accordin...
Text Solution
|
- For the synthesis of ammonia by the reaction N(2)+3H(2)hArr2NH(3) in t...
Text Solution
|
- When the rate of formation of reactants is equal to the rate of format...
Text Solution
|
- Which of the following is a characterisstic of a reversible reaction ?
Text Solution
|
- What is the equilibrium expression for the reaction P(4(s)) + 5O(2(g)...
Text Solution
|
- For the reaction CO(g)+Cl(2)(g) harr COCl(2)(g), K(p)//K(c) is equal t...
Text Solution
|
- The equilibrium constant for the reaction N(2)(g)+O(2)(g) hArr 2NO(g...
Text Solution
|
- Calculate the partial pressure of carbon monoxide from the following d...
Text Solution
|
- Of the following, which change will shift the reaction towards the pro...
Text Solution
|
- Consider the following reversible reactionat equilibrium: 2H(2)O(g) ...
Text Solution
|
- Ammoina carbonate when heated to 200^(@)C gives a mixture of NH(3) and...
Text Solution
|
- Given reaction is 2X((gas)) + Y((gas))hArr2Z((gas)) + 80 Kcal Which ...
Text Solution
|
- The compounds A and B are mixed in equimolar proportion to form the pr...
Text Solution
|
- In the manufacture of ammonia by Haber's process, N(2)(g) +3H(2)(g) ...
Text Solution
|
- The chemical equlibrium of a reversible reaction is not influenced by ...
Text Solution
|
- The equilibrium: P(4)(g)+6Cl(2)(g) hArr 4PCl(3)(g) is attained by ...
Text Solution
|
- The following equilibrium exists in a closed vessel in 1L capacity A(g...
Text Solution
|
- 2 "mole" of PCl(5) were heated in a closed vessel of 2 litre capacity....
Text Solution
|
- Equilibrium constants K(1) and K(2) for the following equilibria NO(...
Text Solution
|