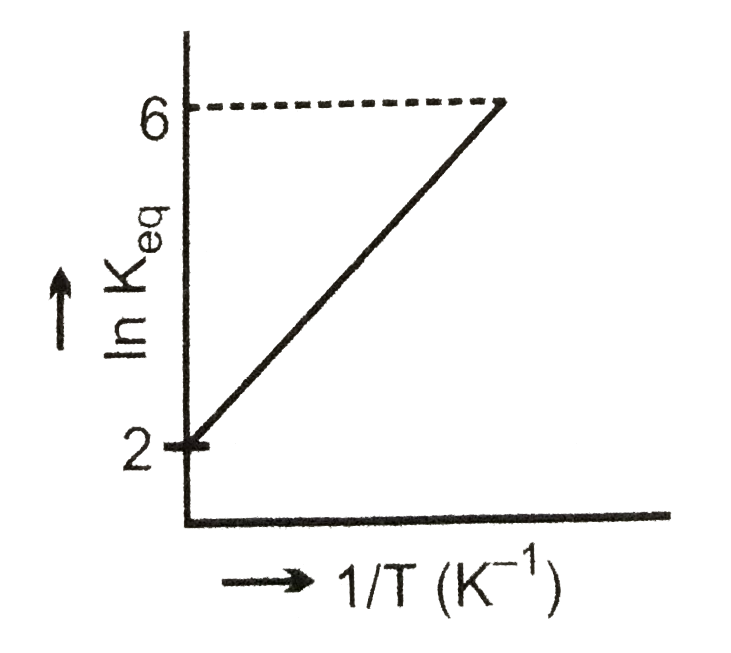A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PHYSICAL AND CHEMICAL EQUILIBRIA
DINESH PUBLICATION|Exercise SELECTED STRAIGHT OBJECTIVE TYPE MCQs|4 VideosPHYSICAL AND CHEMICAL EQUILIBRIA
DINESH PUBLICATION|Exercise MCQs with only one correct answer|24 VideosPHYSICAL AND CHEMICAL EQUILIBRIA
DINESH PUBLICATION|Exercise Multiple CHOICE QUESTIONS [Based on Numerical Problems]|62 VideosP BLOCK ELEMENTS (GROUP 13 AND 14 )
DINESH PUBLICATION|Exercise Straight obj.|17 VideosRATES OF REACTIONS AND CHEMICAL KINETICS
DINESH PUBLICATION|Exercise Ultimate Preparatory Package|29 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-PHYSICAL AND CHEMICAL EQUILIBRIA-REVISION QUESTION FROM COMPETITIVE EXAMS
- The exothermic formation of ClF(3) is represented by thr equation: C...
Text Solution
|
- For the reaction 2NO(2)(g) hArr 2NO(g)+O(2)(g) K(c)=1.8xx10^(-6) at ...
Text Solution
|
- A schematic plot of In K(eq) versus inverse o ftemperature for a react...
Text Solution
|
- An amount of solid NH4HS is placed in a flask already containing ammon...
Text Solution
|
- For the hypothetic reaction, the equilibrium constant (K) values are g...
Text Solution
|
- CaCO(3) hArr CaO + CO(2) reaction in a lime kiln goes to completion b...
Text Solution
|
- A 550 K, the K(c) for the following reaction is 10^(4) mol^(-1) L ...
Text Solution
|
- A + B hArrC + D. If finally the concentrations of A an d B are both eq...
Text Solution
|
- Partial pressure of O(2) in the reaction 2Ag(2)O(s) hArr 4Ag(s)+O(2)...
Text Solution
|
- NH(4)COONH(4)(s) hArr 2NH(3)(g)+CO(2)(g). If equilibrium pressure is 3...
Text Solution
|
- For the reaction N(2(g)) + O(2(g))hArr2NO((g)), the value of K(c) at 8...
Text Solution
|
- For the reaction H(2)(g)+CO(g)hArrCO(g)+H(2)O(g), if the initial conc...
Text Solution
|
- One mole of H(2) and 2 moles of I(2) are taken initially in a two lit...
Text Solution
|
- When hydrogen molecules decompose into its atoms, which conditions giv...
Text Solution
|
- For the reaction, H(2) + I(2)hArr 2HI, K = 47.6 . If the initial numbe...
Text Solution
|
- The equilibrium constant (K(p)) for the decomposition of gaseous H(2)O...
Text Solution
|
- At equilibrium of the reaction , N(2)O(4)(g) hArr 2NO(2)(g) the ob...
Text Solution
|
- According to Le- Chatelier's principile , maximum yiled of NH(3) is ob...
Text Solution
|
- Given the equilibrium system : NH(4)Cl(g) hArr NH(44)^(+)(aq)+Cl^(-)...
Text Solution
|
- Equilivalent amounts of H(2) and I(2) are heated in a closed vessel ti...
Text Solution
|