A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
LIQUID SOLUTION
MOTION|Exercise EXERCISE-2 (LEVEL -I) OBJECTIVE PROBLEMS JEE MAIN|53 VideosLIQUID SOLUTION
MOTION|Exercise EXERCISE-2 (LEVEL -II) MULTIPLE CORRECT JEE ADVANCED|30 VideosLIQUID SOLUTION
MOTION|Exercise EXERCISE-4 (Level-II) PREVIOUS YEAR JEE ADVANCED|19 VideosISOMERISM
MOTION|Exercise Exercise 4|26 VideosMETALLURGY
MOTION|Exercise EXERCISE 4 (LEVEL - II)|17 Videos
Similar Questions
Explore conceptually related problems
MOTION-LIQUID SOLUTION-EXERCISE-1 OBJECTIVE PROBLEMS JEE MAIN
- 10 g of glucose is dissolved in 150 g of water. The mass percentage of...
Text Solution
|
- If 100mL of 1.0M NaOH solution is diluted to 1.0L, the resulting solut...
Text Solution
|
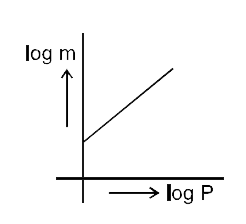
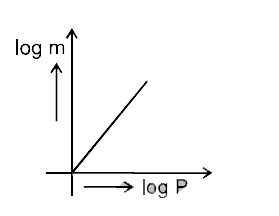
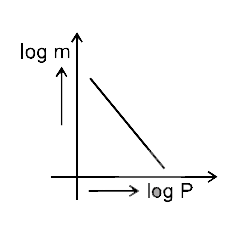
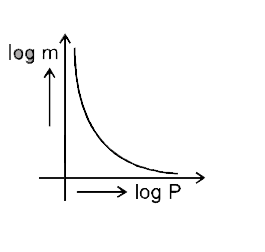
- Which of the following curves represents the Henry's law ?
Text Solution
|
- According to Henry's law, the solubility of a gas in a given volume of...
Text Solution
|
- Some of the following gases are soluble in water due to formation of t...
Text Solution
|
- The boiling point of C(6)H(6), CH(3)OH, C(6)H(5)NH(2) and C(6)H(5)NO(2...
Text Solution
|
- Mole fraction of vapour of A above solution in mixture of A and B(X(A)...
Text Solution
|
- At a given temperature , total vapour pressure (in Torr) of a mixture ...
Text Solution
|
- The vapour pressures of ethanol and methanol are 42.0mmand88.0mmHgresp...
Text Solution
|
- A solution of sulphuric acid in water exhibits:
Text Solution
|
- Binary liquid mixtures which exhibit positive deviations from Raoult’s...
Text Solution
|
- Which of the following is not correct for an ideal solution ?
Text Solution
|
- Which of the following conditions is not correct for ideal solution-
Text Solution
|
- An ideal solution was obtained by mixing methanol and ethanol. If the ...
Text Solution
|
- An ideal solution is that which over the entire range of conce...
Text Solution
|
- Solution distilled without change in composition at a temperature is c...
Text Solution
|
- Which pair shows a contraction in volume on mixing along with evolutio...
Text Solution
|
- Azeotropic mixture of water and HCl boils at 381.5 K. By distilling th...
Text Solution
|
- Which salt may show the same value of vant Hoff factor (i)as that of K...
Text Solution
|
- In which of the following the vant Hoff factor (i) is equal to one:
Text Solution
|