A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
LIQUID SOLUTION
MOTION|Exercise EXERCISE-4 (Level-I) PREVIOUS YEAR JEE MAIN|30 VideosLIQUID SOLUTION
MOTION|Exercise EXERCISE-4 (Level-II) PREVIOUS YEAR JEE ADVANCED|19 VideosLIQUID SOLUTION
MOTION|Exercise EXERCISE-2 (LEVEL -II) MULTIPLE CORRECT JEE ADVANCED|30 VideosISOMERISM
MOTION|Exercise Exercise 4|26 VideosMETALLURGY
MOTION|Exercise EXERCISE 4 (LEVEL - II)|17 Videos
Similar Questions
Explore conceptually related problems
MOTION-LIQUID SOLUTION-EXERCISE-3 SUBJECTIVE JEE ADVANCED
- The vapour pressure of two pure liquids, A and B that form an ideal so...
Text Solution
|
- When the mixture of two immicible liquids (water and nitrobenzene) boi...
Text Solution
|
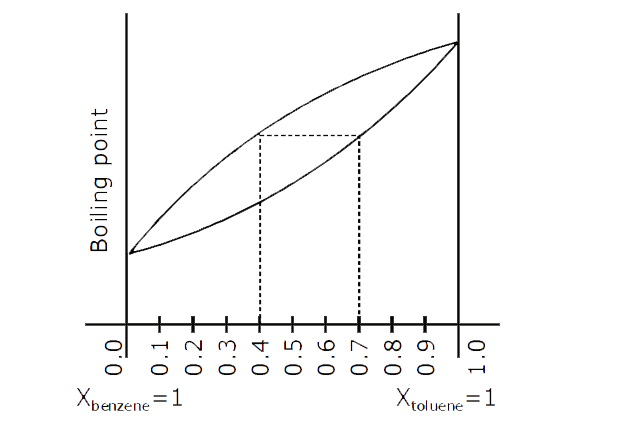
- The following graph represents variation of boiling point with composi...
Text Solution
|
- Boiling point of a mixture of water and nitrobenzene is 99^(@)C, the v...
Text Solution
|
- The vapour pressure of pure liquid solvent A is 0.80 atm. When a non-v...
Text Solution
|
- Calculate the relative lowering in vapour pressure if 100 g of a nonvo...
Text Solution
|
- The degree of dissociation of Ca(NO(3))(2) in a dilute aqueous solutio...
Text Solution
|
- What weight of the non-volatile solute urea needs to be dissolved in 1...
Text Solution
|
- The vapour pressure of an aqueous solution of glucose is 750 mm of Hg ...
Text Solution
|
- Barium ion , CN^(-) and Co^(2+) form an ionic complex . If that comple...
Text Solution
|
- If pK(a)=-"log"K(a)=4, and K(a)=Cx^(2-) then Van't Hoff factor for wea...
Text Solution
|
- pH of 1M HA (weak acid) is 2. Hence van't Hoff factor is -
Text Solution
|
- (a) A solution containing 0.5g of naphithalene in 50g C Cl(4) yield a ...
Text Solution
|
- The boiling point of a solution of 5g of sulphur in 100g of carbon dis...
Text Solution
|
- The vapour pressure of fluorobenzene at t^(@) C is given by the equati...
Text Solution
|
- When 10.6 g of a nonvolatile substance is dissolved in 740 f of ether,...
Text Solution
|
- A solution containing 3.24 g of a nonvolatile nonelectrolyte and 200 g...
Text Solution
|
- An aqueous solution of a non-volatile solute boils at 100.17^(@)C. At ...
Text Solution
|
- Calculate the molal elevation constant , "K"("b") for water and the bo...
Text Solution
|
- 2 g of benzoic acid (C(6)H(5)COOH) dissolved in 25g of benzene shows a...
Text Solution
|