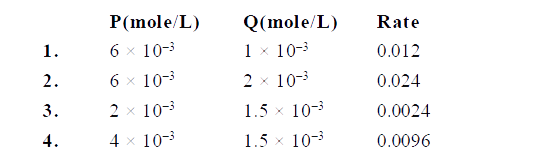Topper's Solved these Questions
CLASSROOM PROBLEMS
MOTION|Exercise Chemical Equilibrium|38 VideosCLASSROOM PROBLEMS
MOTION|Exercise Radioactivity|23 VideosCLASSROOM PROBLEMS
MOTION|Exercise Electrochemistry|22 VideosChemical Kinetics
MOTION|Exercise Exercise - 4 (Level - II) (SUBJECTIVE PROBLEM)|1 VideosCLASSROOM PROBLEMS 1
MOTION|Exercise THERMODYNAMICS|17 Videos
Similar Questions
Explore conceptually related problems
MOTION-CLASSROOM PROBLEMS -Chemical Kinetics
- The stoichiometric equation for the oxidation of bromide ions by hydro...
Text Solution
|
- The stoichiometric equation for the oxidation of bromide ions by hydro...
Text Solution
|
- For a reaction 2P + Q to S , following data were collected. Calculate ...
Text Solution
|
- A 22.4 litre flask contains 0.76 mm of ozone at 25^@C. Calculate the ...
Text Solution
|
- A 22.4 litre flask contains 0.76 mm of ozone at 25^@C. Calculate the r...
Text Solution
|
- The rate of a reaction A + B to products is studied to give following...
Text Solution
|
- For the reaction, 2A + B+C rarr A(2)B+C The rate = k[A][B]^(2) with ...
Text Solution
|
- A to B+ C is a first order reaction Reagent reacts with all A, ...
Text Solution
|
- The first order reaction: Sucrose rarr Glucose + Fructose takes plac...
Text Solution
|
- At room temperature (20^@C) milk turns sour in about 65 hours. In a re...
Text Solution
|
- For a reaction, if effective rate constant k' is given b k=(2k2)/k3(...
Text Solution
|
- For a reaction, if effective rate constant k' is given b k=(2k2)/k3(...
Text Solution
|
- For a reaction, if effective rate constant k' is given b k=(2k2)/k3(...
Text Solution
|
- Two reaction, (I)A rarr Products and (II) B rarr Products, follow firs...
Text Solution
|
- For a reaction A2 + B2 to 2AB, evaluate the energy of activation from ...
Text Solution
|
- A reaction proceeds five times more at 60^(@)C as it does at 30^(@)C. ...
Text Solution
|
- For the reaction A hArr B, DeltaE for the reaction is –33.0 kJ mol^(–...
Text Solution
|
- For the reaction A hArr B, DeltaE for the reaction is –33.0 kJ mol^(–...
Text Solution
|
- A first order reaction A rarr B requires activation energy of 70 kJ mo...
Text Solution
|
- At 380^(@)C , the half-life periof for the first order decompoistion o...
Text Solution
|