Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MOTION-GOC-Exercise - 2 (Level - II)
- A canonical structure will be more stable if (a) it has more number ...
Text Solution
|
- Consider structural formulas A, B and C : underset((A))(H(2)overset(...
Text Solution
|
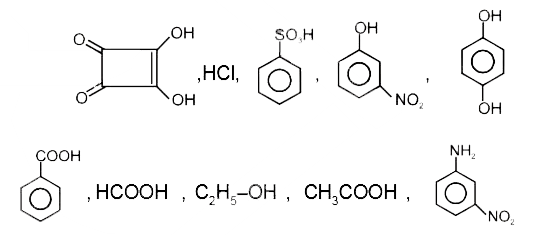
- How many of the following compounds give CO(2) on reaction with NaHCO...
Text Solution
|
- Identify more stable canonical structure in each of the following pair...
Text Solution
|
- Identify more stable canonical structure in each of the following pair...
Text Solution
|
- Identify more stable canonical structure in each of the following pair...
Text Solution
|
- Identify more stable canonical structure in each of the following pair...
Text Solution
|
- Identify more stable canonical structure in each of the following pair...
Text Solution
|
- In the following sets of resonance forms, label the major and minor co...
Text Solution
|
- In the following sets of resonance forms, label the major and minor co...
Text Solution
|
- In the following sets of resonance forms, label the major and minor co...
Text Solution
|
- In the following sets of resonance forms, label the major and minor co...
Text Solution
|
- In the following sets of resonance forms, label the major and minor co...
Text Solution
|
- Which of the following pairs has higher resonance energy: (a). CH(3)...
Text Solution
|
- Which of the following pairs has higher resonance energy : CH(2) = C...
Text Solution
|
- Which of the following pairs has higher resonance energy:
Text Solution
|
- Which of the following pairs has higher resonance energy:
Text Solution
|
- Which of the following pairs has higher resonance energy: and CH(2...
Text Solution
|
- Which of the following pairs has less resonance energy: CO(3)^(2-) "...
Text Solution
|
- Which of the following pairs has less resonance energy: Ө" and "...
Text Solution
|