Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MOTION-GOC-Exercise - 2 (Level - II)
- Which of the following pairs has higher resonance energy:
Text Solution
|
- Which of the following pairs has higher resonance energy:
Text Solution
|
- Which of the following pairs has higher resonance energy: and CH(2...
Text Solution
|
- Which of the following pairs has less resonance energy: CO(3)^(2-) "...
Text Solution
|
- Which of the following pairs has less resonance energy: Ө" and "...
Text Solution
|
- Which of the following pairs has less resonance energy: and CH(...
Text Solution
|
- Which of the following pairs has less resonance energy: o+ " an...
Text Solution
|
- Which of the following pairs has higher resonance energy :
Text Solution
|
- Which of the following pairs has higher resonance energy :
Text Solution
|
- Which of the following pairs has higher resonance energy :
Text Solution
|
- Which of the following pairs has higher resonance energy : CH(2) = C...
Text Solution
|
- Which of the following pairs has higher resonance energy :
Text Solution
|
- underset(("Cyanic acid"))(H - O - C -=N) " "underset(("Isocyanic a...
Text Solution
|
- Ease of ionization to produce carbocation and bromide ion under the tr...
Text Solution
|
- Complete the following reaction
Text Solution
|
- Complete the following reaction
Text Solution
|
- Which one of the following statements is True:
Text Solution
|
- Correct order of rate of hydrolysis or rate of reaction toward AgNO(3)...
Text Solution
|
- Complete the following reaction
Text Solution
|
- Complete the following reaction
Text Solution
|
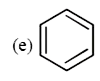 and `CH_(2) = CH - CH = CH - CH = CH_(2)`
and `CH_(2) = CH - CH = CH - CH = CH_(2)`