Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MOTION-GOC-Exercise - 2 (Level - II)
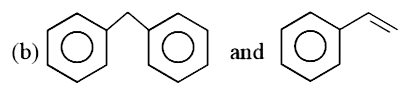
- Which of the following pairs has less resonance energy: o+ " an...
Text Solution
|
- Which of the following pairs has higher resonance energy :
Text Solution
|
- Which of the following pairs has higher resonance energy :
Text Solution
|
- Which of the following pairs has higher resonance energy :
Text Solution
|
- Which of the following pairs has higher resonance energy : CH(2) = C...
Text Solution
|
- Which of the following pairs has higher resonance energy :
Text Solution
|
- underset(("Cyanic acid"))(H - O - C -=N) " "underset(("Isocyanic a...
Text Solution
|
- Ease of ionization to produce carbocation and bromide ion under the tr...
Text Solution
|
- Complete the following reaction
Text Solution
|
- Complete the following reaction
Text Solution
|
- Which one of the following statements is True:
Text Solution
|
- Correct order of rate of hydrolysis or rate of reaction toward AgNO(3)...
Text Solution
|
- Complete the following reaction
Text Solution
|
- Complete the following reaction
Text Solution
|
- Aromatic compounds are:
Text Solution
|
- Which of the following reactions give aromatic compound ?
Text Solution
|
- Write stability order of following intermediates: (a)CH(3) - overset...
Text Solution
|
- Write stability order of following intermediates:
Text Solution
|
- Write stability order of following intermediates:
Text Solution
|
- Write stability order of following intermediates: (a)CH(3)- overset(...
Text Solution
|