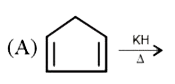
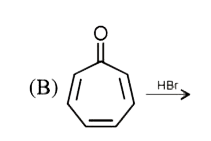
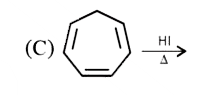
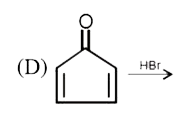
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MOTION-GOC-Exercise - 2 (Level - II)
- Complete the following reaction
Text Solution
|
- Aromatic compounds are:
Text Solution
|
- Which of the following reactions give aromatic compound ?
Text Solution
|
- Write stability order of following intermediates: (a)CH(3) - overset...
Text Solution
|
- Write stability order of following intermediates:
Text Solution
|
- Write stability order of following intermediates:
Text Solution
|
- Write stability order of following intermediates: (a)CH(3)- overset(...
Text Solution
|
- Write stability order of following intermediates:
Text Solution
|
- Write stability order of following intermediates:
Text Solution
|
- Write stability order of following intermediates: (a)HC -= overset(T...
Text Solution
|
- Write stability order of following intermediates:
Text Solution
|
- Write stability order of following intermediates:
Text Solution
|
- Write stability order of following intermediates:
Text Solution
|
- Arrange the following in correct order of their stability ? (I) CH-=ov...
Text Solution
|
- Compare the stability of the following free Radical. (a) CH(3) - ove...
Text Solution
|
- Write stability order of following intermediates:
Text Solution
|
- Write stability order of following intermediates:
Text Solution
|
- Write stability order of following intermediates:
Text Solution
|
- Write stability order of following intermediates:
Text Solution
|
- Write stability order of following intermediates: (a)overset(Ө)CH(2)...
Text Solution
|