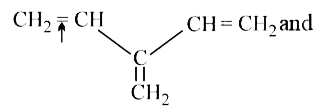
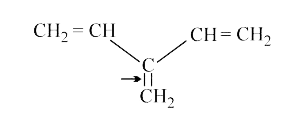
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MOTION-GOC-Exercise - 2 (Level - II)
- In which of the following pairs, indicated bond having less bond disso...
Text Solution
|
- In which of the following pairs, indicated bond having less bond disso...
Text Solution
|
- In which of the following pairs, indicated bond having less bond disso...
Text Solution
|
- In which of the following pairs, indicated bond having less bond disso...
Text Solution
|
- In which of the following pairs, indicated bond having less bond disso...
Text Solution
|
- In which of the following pairs, indicated bond having less bond disso...
Text Solution
|
- Compare the C–N bond-length in the following species:
Text Solution
|
- Which of the following statements would be true about this compound:
Text Solution
|
- Choose the more stable alkene in each of the following pairs. Explain ...
Text Solution
|
- Choose the more stable alkene in each of the following pair. Explain y...
Text Solution
|
- Choose the more stable alkene in each of the following pairs. Explain ...
Text Solution
|
- Consider the given reaction : In the above reaction which one of ...
Text Solution
|
- Compare heat of hydrogenation (Decreasing order) heat of hydrogenati...
Text Solution
|
- Compare heat of hydrogenation (Decreasing order)
Text Solution
|
- Compare heat of hydrogenation (Decreasing order)
Text Solution
|
- Compare heat of hydrogenation (Decreasing order)
Text Solution
|
- Compare heat of hydrogenation (Decreasing order)
Text Solution
|
- (I) Stability order and (II) heat of hydrogenation orders.
Text Solution
|
- (I) Stability order and (II) heat of hydrogenation orders.
Text Solution
|
- Among the following pairs identify the one which gives higher heat of ...
Text Solution
|