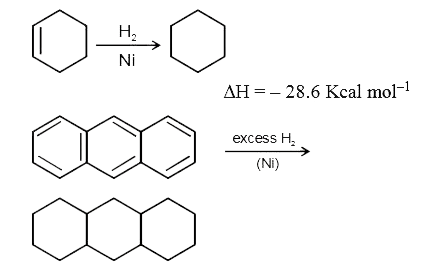
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MOTION-GOC-Exercise - 2 (Level - II)
- Match each alkene with the appropriate heat of combustion: Heats of ...
Text Solution
|
- Match each alkene with the appropriate heat of combustion: Heats of ...
Text Solution
|
- Match each alkene with the appropriate heat of combustion: Heats of ...
Text Solution
|
- Write increasing order of heat of hydrogenation :
Text Solution
|
- Write increasing order of heat of hydrogenation :
Text Solution
|
- Write increasing order of heat of hydrogenation :
Text Solution
|
- Write increasing order of heat of hydrogenation :
Text Solution
|
- Write increasing order of heat of hydrogenation :
Text Solution
|
- Write increasing order of heat of hydrogenation :
Text Solution
|
- Give decreasing order of heat of combustion (HOC):
Text Solution
|
- Give decreasing order of heat of combustion (HOC):
Text Solution
|
- Give decreasing order of heat of combustion (HOC):
Text Solution
|
- Arrange in order of C–H bond energy
Text Solution
|
- Use the following data to answer the questions below: DeltaH = - ...
Text Solution
|
- Arrange the given phenols in their decreasing order of acidity: (I) ...
Text Solution
|
- Which one of the following is the most acidic?
Text Solution
|
- Which one of the following phenols will show highest acidity?
Text Solution
|
- Which of the following is weakest acid?
Text Solution
|
- Arrange pH of the given compounds in decreasing order: (1) Phenol ...
Text Solution
|
- Consider the following compound : Which of the above compounds react...
Text Solution
|