Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 34-CHEMISTRY
- Analysis show that nickel oxide consists of nickel ion with 96% ions h...
Text Solution
|
- In which compound does vanadium have an oxidation number of +4?
Text Solution
|
- Among the following, the species that is both paramagnetic and coloure...
Text Solution
|
- Select the correct statement about elements of group 15th.
Text Solution
|
- Which of the following compounds does not give N(2) on heating?
Text Solution
|
- A brown coloured mixture of two gases is obtained by the reduction of ...
Text Solution
|
- Which one of the following compounds on reduction with LiAIH(4) yeilds...
Text Solution
|
- An alkane of molecular weight 86 g/mol on monochlorination gives two p...
Text Solution
|
- The osmotic pressures of equimolar solutions of urea,BaCl(2)" and "Al...
Text Solution
|
- Structurally biodegradable detergent should contain :
Text Solution
|
- Which one among the following is the best reagent for the conversion o...
Text Solution
|
- Which of the following reactions will yield propan-2-ol ? Select the r...
Text Solution
|
Text Solution
|
- Both Co^(3+) and Pt^(4+) have a coordination number of six. Which of t...
Text Solution
|
- The degree of dissociation of a weak monoprotic acid of concentration ...
Text Solution
|
- Half - life period of the radioactive element A is 10 days. Amount of ...
Text Solution
|
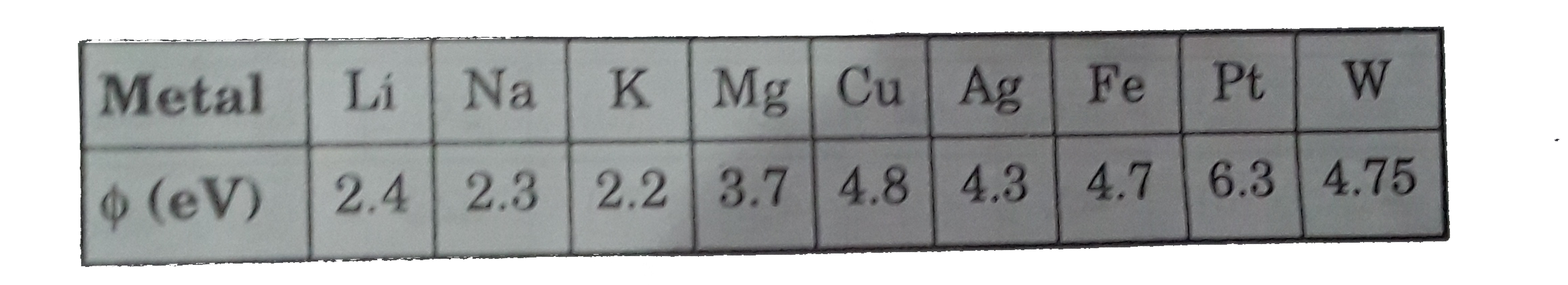
- The work function (phi) of some metals is listed below . The number of...
Text Solution
|
- The decahydrate form of sodium carbonate i.e. washing soda on standing...
Text Solution
|
- A current of 5.0 A flows for 4.0 h through an electrolytic cell contai...
Text Solution
|
- Two moles of an ideal monoatomic gas at 5 bar and 300 K are expanded i...
Text Solution
|