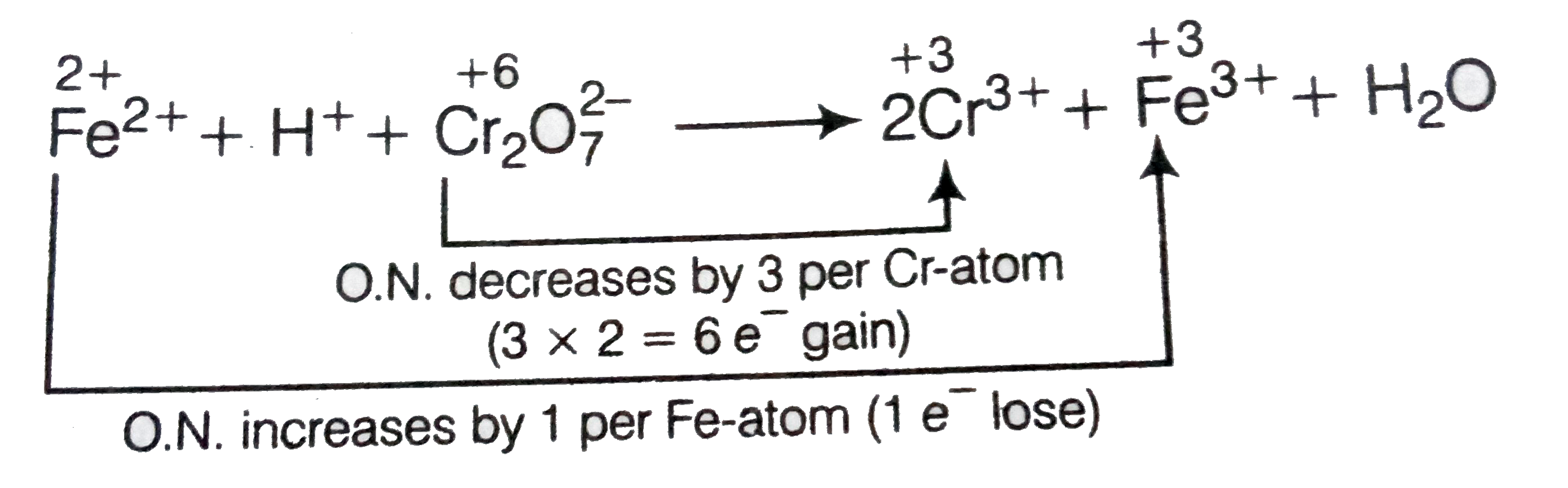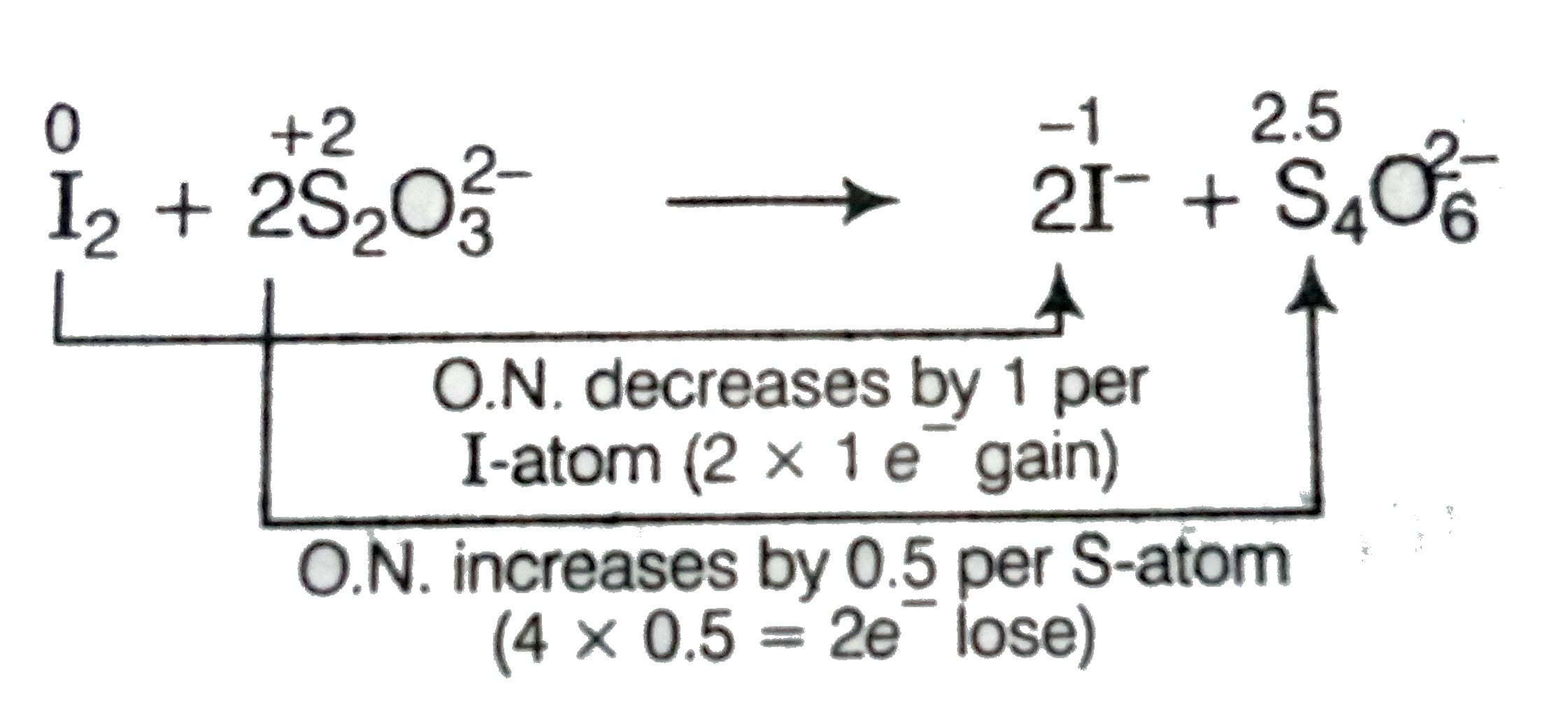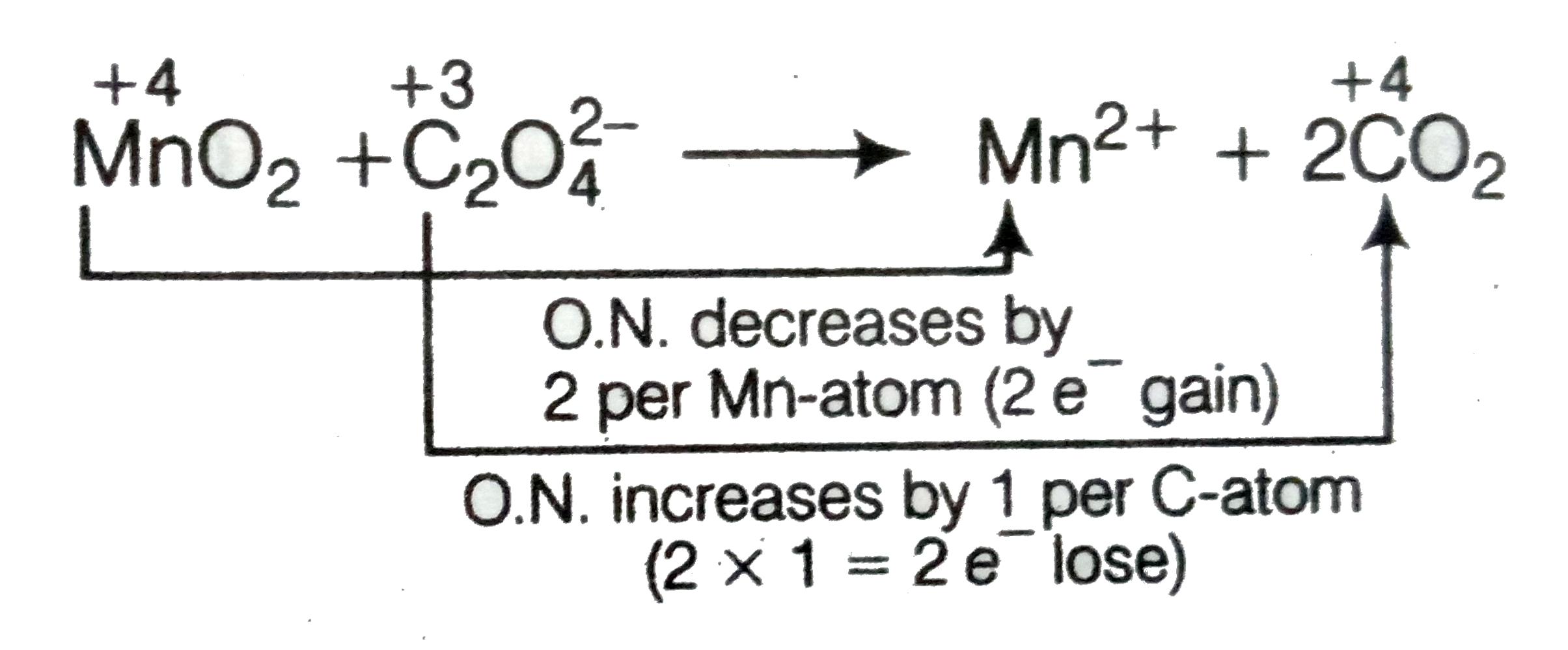Text Solution
Verified by Experts
Topper's Solved these Questions
REDOX REACTIONS
NCERT EXEMPLAR|Exercise ASSERTION AND REASON|4 VideosREDOX REACTIONS
NCERT EXEMPLAR|Exercise LONG ANSWER TYPE QUESTIONS|6 VideosREDOX REACTIONS
NCERT EXEMPLAR|Exercise LONG ANSWER TYPE QUESTIONS|6 VideosORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES
NCERT EXEMPLAR|Exercise Long Answer type question|6 VideosSOME BASIC CONCEPTS OF CHEMISTRY
NCERT EXEMPLAR|Exercise All Questions|45 Videos
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-REDOX REACTIONS-SHORT ANSWER TYPE QUESTIONS
- The reaction Cl(2)(g)+20H^(-)(aq)toClO^(-)(aq)+Cl^(-)(aq)+H(2)O(l) rep...
Text Solution
|
- MnO(4)^(2-) undergoes disproportionation reaction in acidic medium but...
Text Solution
|
- PbO and PbO(2) react with HCl according to following chemical equation...
Text Solution
|
- Nitric acid is an oxidising agent and reacts with PbO but it does not ...
Text Solution
|
- Balance the following by ion electron method in acidic medium. CIO(3...
Text Solution
|
- Calculate the oxidation number of phosphorus in the following species....
Text Solution
|
- Calculate the oxidation number of each sulphur atom in the following c...
Text Solution
|
- Balance the following equations : a. Fe^(3+) + Sn^(+2) rarr Sn^(4+) ...
Text Solution
|
- Identify the redox reaction out of the following reacitons and identif...
Text Solution
|
- Balance the following ionic equation. MnO(4)^(-)+H^(+)+Br^(-)toMn^(2...
Text Solution
|
- Match column I and column II for the oxidation states of the central a...
Text Solution
|
- Match the items in column I with relevant items in column II
Text Solution
|