A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
NCERT EXEMPLAR|Exercise LONG ANSWER TYPE QUESTIONS|6 VideosREDOX REACTIONS
NCERT EXEMPLAR|Exercise SHORT ANSWER TYPE QUESTIONS|12 VideosORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES
NCERT EXEMPLAR|Exercise Long Answer type question|6 VideosSOME BASIC CONCEPTS OF CHEMISTRY
NCERT EXEMPLAR|Exercise All Questions|45 Videos
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-REDOX REACTIONS-ASSERTION AND REASON
- Assertion (A) Among halogens fluorine is the best oxidation. Reason ...
Text Solution
|
- Assertion (A) In the reaction between potassium permanganate and potas...
Text Solution
|
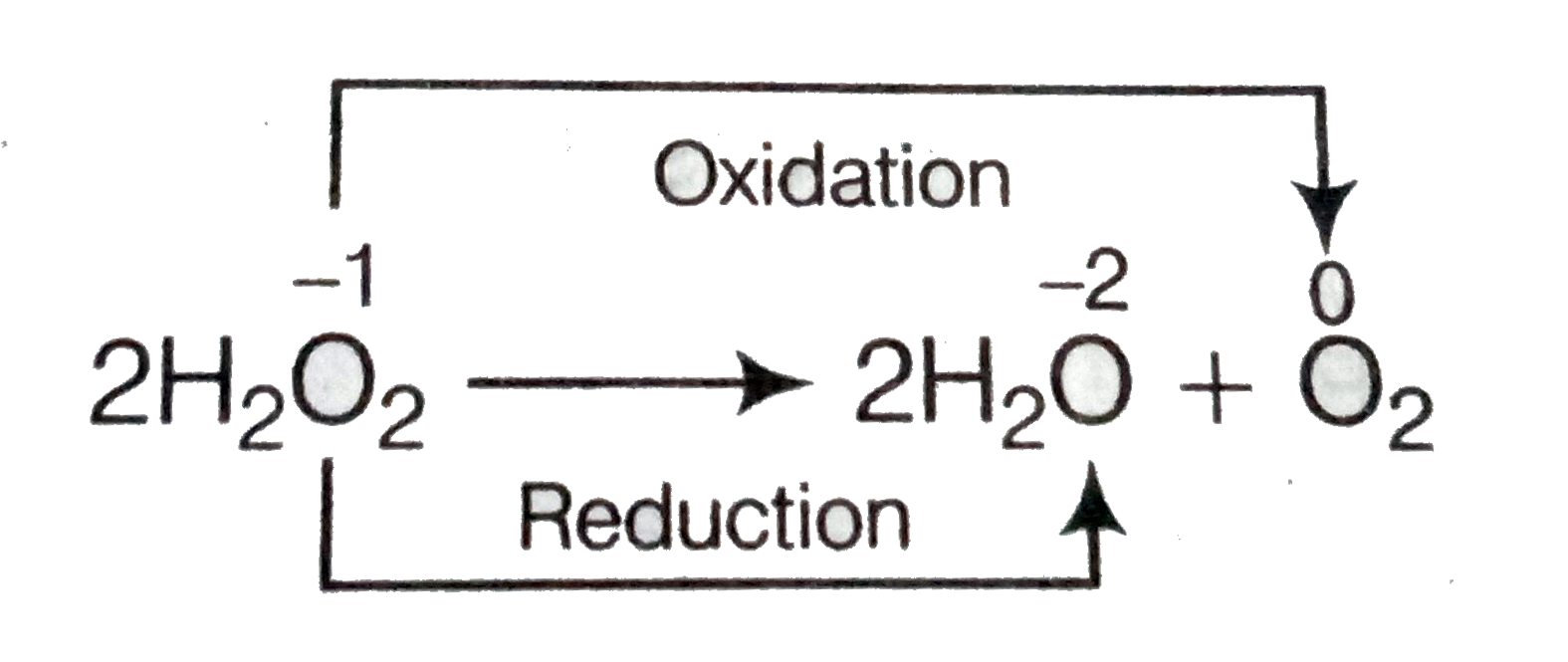
- Assertion (A) The decomposition of hydrogen peroxide to form water and...
Text Solution
|
- Assertion (A) Redox couple is the combination of oxidised and reduced ...
Text Solution
|